WEST CHESTER, Pa., Feb. 26, 2025 /PRNewswire/ -- Centinel Spine®, LLC ("the Company"), the leading global medical device company focused exclusively on treating cervical and lumbar spinal disease with the most complete and clinically-proven total disc replacement (TDR) technology platform in the world (prodisc®), today announced that is has entered into a senior … [Read more...] about Centinel Spine® Refinances Existing Senior Secured Term Loan Facility with SLR Capital Partners
NEWS
SIGNUS Receives FDA Approval for CYLOX® ST cage and plate system
Alzenau, 26/02/2025 – SIGNUS Medizintechnik GmbH is proud to announce the successful FDA approval of the CYLOX® ST cage and plate system. This FDA clearance marks a significant milestone in the expansion of our product range in the US market as well as in advancing spinal care, ensuring improved patient outcomes and procedural efficiency. SIGNUS is dedicated to the … [Read more...] about SIGNUS Receives FDA Approval for CYLOX® ST cage and plate system
Life Spine Announces First Surgical Cases of ARx® SAI Spinal Fixation System
HUNTLEY, Ill.--(BUSINESS WIRE)--Life Spine, Inc., a medical device company that designs, develops, manufactures and markets products for the surgical treatment of spinal disorders, announced today the first surgical cases of ARx SAI (Sacral Alar Iliac) Spinal Fixation System. The ARx SAI Spinal Fixation System represents the next evolution in posterior fixation technology. … [Read more...] about Life Spine Announces First Surgical Cases of ARx® SAI Spinal Fixation System
Orthofix Reports Fourth Quarter and Full-Year 2024 Results and Provides 2025 Financial Guidance
LEWISVILLE, Texas--(BUSINESS WIRE)--Orthofix Medical Inc. (NASDAQ:OFIX), a leading global medical technology company, today reported its financial results for the fourth quarter and full-year ended December 31, 2024, and provided full-year 2025 financial guidance. Highlights Fourth quarter 2024 net sales were $215.7 million, an increase of 8% on a reported and constant … [Read more...] about Orthofix Reports Fourth Quarter and Full-Year 2024 Results and Provides 2025 Financial Guidance
Alphatec Today: Where It Stands and Where It’s Heading?
Alphatec Holdings, Inc. (Nasdaq: ATEC) is executing an ambitious expansion plan based on innovation, talent acquisition, and investor confidence. The company’s participation in key events, use of financial incentives, and commitment to advanced technological solutions demonstrate its determination to strengthen its position in the highly competitive spine surgery market. In … [Read more...] about Alphatec Today: Where It Stands and Where It’s Heading?
We are proud to announce that Syntropiq will once again sponsor SPINEMarketGroup in 2025!
Thank you, Syntropiq! On behalf of the SPINEMarketGroup team, we sincerely appreciate your continued support as a PLATINUM sponsor for 2025.We are very proud to continue this journey together for another year! About Syntropiq We are a privately held spine interbody implant and surface technology company. Our team members with rich experience more than 25 … [Read more...] about We are proud to announce that Syntropiq will once again sponsor SPINEMarketGroup in 2025!
We are proud to announce that NGMedical will once again sponsor SPINEMarketGroup in 2025!
Thank you, NGMedical! On behalf of the SPINEMarketGroup team, we sincerely appreciate your continued support as a PLATINUM sponsor for 2025.We are very proud to continue this journey together for another year! About NGMedical NGMedical combines science and innovation and stands for the development and production of highly innovative implants for spine surgery. In … [Read more...] about We are proud to announce that NGMedical will once again sponsor SPINEMarketGroup in 2025!
Why Didn’t Globus Medical’s Stock Rise After Their Excellent Financial Results?
Globus Medical's results published yesterday were excellent, with a 6.6% increase in fourth-quarter sales for 2024 and an impressive 60.6% annual growth in sales. However, despite these solid numbers, the company’s stock did not experience the expected rise and dropped slightly. Full Year 2024: So What Happened Yesterday? While these numbers are undeniably strong, … [Read more...] about Why Didn’t Globus Medical’s Stock Rise After Their Excellent Financial Results?
Globus Medical Reports Fourth Quarter and Full Year 2024 Results
AUDUBON, Pa., Feb. 20, 2025 (GLOBE NEWSWIRE) -- Globus Medical, Inc. (NYSE: GMED), a leading musculoskeletal technology company, today announced its financial results for the fourth quarter and year ended December 31, 2024. Fourth Quarter 2024: Full Year 2024: “I’m proud of our team at Globus Medical, delivering incredible results … [Read more...] about Globus Medical Reports Fourth Quarter and Full Year 2024 Results
Accelus to Showcase Advanced Expandable Spinal Implant Technologies at 2025 Spine Summit
PALM BEACH GARDENS, FL, UNITED STATES, February 19, 2025 /EINPresswire.com/ -- Accelus, a privately held medical technology company committed to becoming the global market leader in expandable spinal implant technologies, today announced its participation in the 2025 Spine Summit, taking place February 20–23 at the JW Marriott Tampa Water Street in Tampa, Florida. The Spine … [Read more...] about Accelus to Showcase Advanced Expandable Spinal Implant Technologies at 2025 Spine Summit
Life Spine Announces FDA 510(k) Clearance for the GRUVE® + Cervical Plating System
HUNTLEY, Ill.--(BUSINESS WIRE)--Life Spine, Inc., a medical device company that designs, develops, manufactures and markets products for the surgical treatment of spinal disorders, announced today that it has received clearance from the U.S. Food & Drug Administration (FDA) to market GRUVE + Cervical Plating System. GRUVE + (plus) Cervical Plating System establishes a … [Read more...] about Life Spine Announces FDA 510(k) Clearance for the GRUVE® + Cervical Plating System
Groundbreaking Biportal Endoscopic Spinal Fusion Performed Using Mazor Robotic Guidance
PHOENIX, Feb. 17, 2025 / – In a landmark achievement in spinal surgery, Dr. Andrew Chung performed an unprecedented biportal endoscopic spinal fusion at Banner Del E Webb Hospital on January 27, 2025. This pioneering procedure integrated Amplify Surgical's dualX®Slim interbody fusion implant with the dualPortal® endoscopic technique, while leveraging Medtronic's Mazor robotic … [Read more...] about Groundbreaking Biportal Endoscopic Spinal Fusion Performed Using Mazor Robotic Guidance
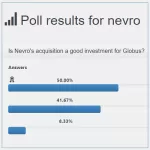
What do voters think (Poll results) about Globus’s acquisition of Nevro?
Last week, after the announcement of Globus's acquisition of Nevro, we wanted to learn the opinion of our visitors about this move. To do so, we published a poll with the following question: Is Nevro's acquisition a good investment for Globus? and the following answers:a) I'm not sure, it will depend on integration and executionb) Yes, it complements its portfolio and will … [Read more...] about What do voters think (Poll results) about Globus’s acquisition of Nevro?
We are proud to announce that LfC will once again sponsor SPINEMarketGroup in 2025!
Thank you, LfC! On behalf of the SPINEMarketGroup team, we sincerely appreciate your continued support as a PLATINUM sponsor for 2025.We are very proud to continue this journey together for another year! About LfC LfC is a polish company who have achieved a leading position in the design and manufacture of surgical equipment used in spinal treatment in orthopaedics and … [Read more...] about We are proud to announce that LfC will once again sponsor SPINEMarketGroup in 2025!
Expanding Innovations to Showcase Cutting-Edge Expandable Cage Technology at Spine Summit 2025
MOUNTAIN VIEW, Calif., Feb. 13, 2025 /PRNewswire/ -- Expanding Innovations™ (EI), an emerging leader in expandable implant technology for spine surgery, is proud to announce its participation in the 2025 AANS/CNS Joint Section Spine Summit in Tampa, FL, from February 20-23. Attendees are invited to visit the Expanding Innovations booth to discover how … [Read more...] about Expanding Innovations to Showcase Cutting-Edge Expandable Cage Technology at Spine Summit 2025
Stryker’s Spine Exit: What It Means for Europe, Asia, and Beyond
Stryker, a leading player in the medical device industry, has made a strategic shift by selling its U.S. spine implant business to Viscogliosi Brothers, LLC (VB), an investment firm specializing in the neuro-musculoskeletal sector. This acquisition will lead to the creation of a new entity, VB Spine, LLC. The deal, expected to close in the first half of 2025, also includes a … [Read more...] about Stryker’s Spine Exit: What It Means for Europe, Asia, and Beyond
Update:Stay Tuned Next Week! Is Globus Medical the New Leader in the Spine Market?
Recently, we have received several requests to publish an article with data showing whether Globus Medical has already surpassed Medtronic to become the number one player in the spine market. Although Globus posted exceptional year-end 2024 results earlier in January, we are awaiting the upcoming report on February 20 to conduct a thorough analysis and provide you with the most … [Read more...] about Update:Stay Tuned Next Week! Is Globus Medical the New Leader in the Spine Market?
Certain Nano Surface Technology Assets Developed by Nanovis Have Been Acquired by Medtronic
COLUMBIA CITY, Ind., Feb. 12, 2025 /PRNewswire/ -- Nanovis, a leading developer and provider of nano surface technology that improves the biologic fixation of orthopedic, spine, and dental implants, announced today that certain of its nano surface technology assets and certain of its rights to intellectual property assets related to Sites Medical's … [Read more...] about Certain Nano Surface Technology Assets Developed by Nanovis Have Been Acquired by Medtronic
Over 10,000 Procedures Completed in U.S. with Centinel Spine’s Match-the-Disc™ prodisc® Cervical Total Disc Replacement System
WEST CHESTER, Pa., Feb. 11, 2025 /PRNewswire/ -- Centinel Spine®, LLC ("the Company"), the leading global medical device company focused exclusively on treating cervical and lumbar spinal disease with the most complete and clinically-proven total disc replacement (TDR) technology platform in the world (prodisc®), today announced the completion of the 10,000th case in … [Read more...] about Over 10,000 Procedures Completed in U.S. with Centinel Spine’s Match-the-Disc™ prodisc® Cervical Total Disc Replacement System