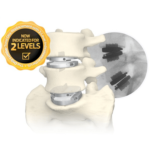
ST. PAUL, MN.– Spineology Inc., the pioneers of Conform and Expand™ Technology, today announced the launch of its latest innovation, and most advanced patient specific solution yet: OptiMesh Align (Align). Built on the proven foundation of OptiMesh®, Align is uniquely engineered to provide real time, in-situ, independent anterior and posterior implant expansion resulting in a … [Read more...] about Spineology® Announces the Launch of OptiMesh Align™
NEWS
Nexxt Spine Announces Key Updates to Executive Leadership Team
NOBLESVILLE, IN, UNITED STATES, June 9, 2025 /EINPresswire.com/ -- Nexxt Spine, LLC, a privately held manufacturer of spinal implants and instrumentation, announced key updates to the Company’s Executive Leadership Team. Ryan Campion has been promoted to Chief Strategy and Development Officer (CSDO); Tom O’Hara has been promoted to Chief Commercial Officer (CCO); Chief People … [Read more...] about Nexxt Spine Announces Key Updates to Executive Leadership Team
(UPDATED 2025): Starting Spine Endoscopy? Best Companies to Consider and Why the Market Is Heating Up!
Endoscopic spinal surgery is becoming an important alternative to traditional open spine surgery by offering less invasive options with faster recovery and fewer complications. This technique uses very small incisions, usually under an inch, along with specialized tubular retractors and a high-definition endoscope to provide surgeons with a clear, magnified view of the spine. … [Read more...] about (UPDATED 2025): Starting Spine Endoscopy? Best Companies to Consider and Why the Market Is Heating Up!
Nanox Receives MDR CE Mark for HealthOST, an Advanced AI-Powered Software for Spine Assessment
PETACH TIKVA, Israel, June 05, 2025 (GLOBE NEWSWIRE) -- NANO-X IMAGING LTD ("Nanox" or the "Company", Nasdaq: NNOX), an innovative medical imaging technology company, today announced that its deep-learning medical imaging analytics subsidiary, Nanox AI Ltd, has received EU MDR CE (Conformité Européenne) mark certification for HealthOST, an SaMD (Software as a medical device) … [Read more...] about Nanox Receives MDR CE Mark for HealthOST, an Advanced AI-Powered Software for Spine Assessment
Centinel Spine® Continues to Innovate in Lumbar Total Disc Replacement through the Release of New prodisc® L Instrumentation
WEST CHESTER, Pa., June 5, 2025 /PRNewswire/ -- Centinel Spine®, LLC ("the Company"), the leading global medical device company focused exclusively on treating cervical and lumbar spinal disease with the most complete and clinically-proven total disc replacement technology platform in the world (prodisc®), today announced the release of new instrumentation for its … [Read more...] about Centinel Spine® Continues to Innovate in Lumbar Total Disc Replacement through the Release of New prodisc® L Instrumentation
Avicenna.AI Secures CE Mark for Two Spine-Focused Medical Imaging AI Tools
MARSEILLE, France, June 5, 2025 /PRNewswire/ -- Medical imaging AI company Avicenna.AI today announced that it has received CE Mark certification for CINA-VCF Quantix, a new AI algorithm designed to detect and assess unsuspected vertebral compression fractures (VCFs) in patients undergoing CT scans for unrelated conditions. The company also secured CE … [Read more...] about Avicenna.AI Secures CE Mark for Two Spine-Focused Medical Imaging AI Tools
Spineart Announces Full-Market Launch of Scarlet ® AC-Ti Secured Anterior Cervical Cage in the US
GENEVA, June 5, 2025 /PRNewswire/ -- Spineart announces the full-market launch of its SCARLET® AC-Ti secured anterior cervical cage in the United States. Following 510(k) clearance in May 2024, SCARLET® AC-Ti has been extensively evaluated by clinical experts and this launch signifies the Company's continued commitment to US product … [Read more...] about Spineart Announces Full-Market Launch of Scarlet ® AC-Ti Secured Anterior Cervical Cage in the US
Highridge Medical Completes the Sale of its Bone Healing Division to Streamline Strategic Focus and Innovation in Spine
WESTMINSTER, Colo., June 04, 2025 (GLOBE NEWSWIRE) -- Highridge Medical (“Highridge” or the “Company”), one of the world’s largest privately held Spine companies, has completed the sale of EBI, its Bone Healing division, to Avista Healthcare Partners. The transaction aligns with the Company’s strategy to exclusively focus on Spine and drive long-term growth through dedicated … [Read more...] about Highridge Medical Completes the Sale of its Bone Healing Division to Streamline Strategic Focus and Innovation in Spine
CMR Surgical Considers Potential $4 Billion Sale Amid Global Expansion of Versius Robot
Cambridge, UK – British medical technology company CMR Surgical, developer of the next-generation Versius surgical robot, is reportedly exploring a potential sale valued at around $4 billion, according to sources familiar with the matter. The move comes at a pivotal moment as the company expands globally and strengthens its foothold in the highly competitive robotic-assisted … [Read more...] about CMR Surgical Considers Potential $4 Billion Sale Amid Global Expansion of Versius Robot
Ti/PEEK Cages with Screw Fixation in ACDF: Redmond® C Clinical Data Review
Cervical spine surgery has seen significant advances in recent years, particularly in the design and performance of interbody cages used in anterior cervical discectomy and fusion (ACDF) procedures. A recent prospective study titled "Integral Fixation Titanium/Polyetheretherketone Cages for Cervical Arthrodesis: Evolution of Cage Design and Early Radiological Outcomes and … [Read more...] about Ti/PEEK Cages with Screw Fixation in ACDF: Redmond® C Clinical Data Review
Insert pedicle screws more precisely and safely without a robot or navigation system? Which are the alternatives in 2025?
Placing pedicle screws in the spine demands precision, speed, and safety. These three principles are critical to minimizing complications, avoiding neurological injury, and achieving optimal clinical outcomes. In recent years, we've seen a technological shift with the emergence of robotic systems and advanced imaging solutions, offering greater accuracy and … [Read more...] about Insert pedicle screws more precisely and safely without a robot or navigation system? Which are the alternatives in 2025?
All Charges Dismissed Against Dr. Kingsley R. Chin and His Companies as DOJ Case Concludes
After a multi-year DOJ investigation, all criminal charges against Dr. Kingsley R. Chin and his medical device company, SpineFrontier, have been dismissed, allowing him to focus on transforming spine care and improving patient outcomes. FORT LAUDERDALE, Fla., May 30, 2025 /PRNewswire/ -- All criminal charges have been dismissed against Dr. Kingsley R. Chin and his … [Read more...] about All Charges Dismissed Against Dr. Kingsley R. Chin and His Companies as DOJ Case Concludes
Thinking About a Spine Robot? Your 2025 Guide to the Best Models, What’s Coming, Why You Need One, and Which Is Truly the Best?
Robotic surgery is experiencing unstoppable growth across all surgical fields, and spine is at the forefront of this transformation. Robotic systems like Medtronic’s Mazor X and Globus Medical’s ExcelsiusGPS are changing the way spinal procedures are performed—bringing sub-millimetric accuracy, greater control, and enhanced safety to the OR. These platforms are especially … [Read more...] about Thinking About a Spine Robot? Your 2025 Guide to the Best Models, What’s Coming, Why You Need One, and Which Is Truly the Best?
Reflections on the Four Pillars That Truly Retain Talent: Our Perspective
This article rightly points out that attracting and retaining talent isn’t achieved through stylish offices or trendy events but through strong and genuine foundations. It emphasizes four essential elements: a clear and meaningful vision, leadership that inspires, fair compensation, and a culture that genuinely recognizes and empowers individuals. It also highlights the risks … [Read more...] about Reflections on the Four Pillars That Truly Retain Talent: Our Perspective
Accelus Secures New Growth Financing To Support Continued Commercial Expansion and Pipeline Innovation
PALM BEACH GARDENS, Fla.--(BUSINESS WIRE)--Accelus (“The Company”), a privately held medical technology company committed to becoming the global market leader in expandable spinal implant technologies, today announced the closing of a new equity financing round led by Concord Health Partners, a healthcare-focused investment firm. The capital will support Accelus’s continued … [Read more...] about Accelus Secures New Growth Financing To Support Continued Commercial Expansion and Pipeline Innovation
Xtant Medical Announces the Launch of OsteoFactor Pro™
BELGRADE, Mont., May 28, 2025 /PRNewswire/ -- Xtant Medical Holdings, Inc. (NYSE American: XTNT), a global medical technology company focused on surgical solutions for the treatment of spinal, orthopedic and wound care disorders, today announced the commercial launch of OsteoFactor Pro™, a naturally occurring cocktail of allogeneic growth factors engineered to … [Read more...] about Xtant Medical Announces the Launch of OsteoFactor Pro™
See All AI Raises $33 Million to Bring Revolutionary AI-Based Sliceable Intraoperative 3D Visualization and Advanced Navigation to Global Healthcare
Groundbreaking platform generates high-resolution 3D models with 1mm cross-sectional slices from as few as two intraoperative fluoroscopic X-ray images, eliminates the need for pre-operative scans, and delivers precise intraoperative device tracking using visible light. Recently completed a second milestone product demonstration for the surgical and investor communities, with … [Read more...] about See All AI Raises $33 Million to Bring Revolutionary AI-Based Sliceable Intraoperative 3D Visualization and Advanced Navigation to Global Healthcare
Top Expandable Cages of 2025: Which Lumbar Implants Are Really Leading the Way?!
Over the past decade, posterior lumbar interbody fusion (PLIF/TLIF) has evolved—driven in part by the development of expandable interbody cages that adapt in real-time to patient anatomy. These implants have gained popularity among spine surgeons for their ability to minimize neural retraction, improve endplate contact, and restore lumbar alignment, all while integrating … [Read more...] about Top Expandable Cages of 2025: Which Lumbar Implants Are Really Leading the Way?!
MSKai Spine Imaging Software Receives FDA 510(k) Clearance for Clinical Use
JACKSON, Miss., May 22, 2025 /PRNewswire/ -- MSKai, an advanced image identification and post-processing software platform for spine imaging, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA), authorizing its use by physicians and radiologists for lumbar spine MRI analysis. The FDA determined that MSKai is substantially equivalent to … [Read more...] about MSKai Spine Imaging Software Receives FDA 510(k) Clearance for Clinical Use