NGMedical GmbH, a leading innovator in spinal motion preservation technology, reports significant growth in 2025 NONNWEILER, SAARLAND, GERMANY, February 23, 2026 /EINPresswire.com/ -- NGMedical reports another year of double-digit growth. In 2025 revenue grew 43 % above the previous year after 90 % growth in 2024. The biggest contribution to the successful development is … [Read more...] about NGMedical Reports Successful 2025 and Sales Milestone for Move®-C
NEWS
DePuy Synthes for Sale: Who Could Actually Buy the Orthopedic Giant?
J&J dropped the news yesterday that they're looking into selling off DePuy Synthes. Honestly, we've been calling this move ever since the spin-off wrapped up. But here's what everyone in the industry is really wondering about: Who could realistically buy an asset of this size and complexity? A rare… and complicated opportunity DePuy Synthes remains one of the leading … [Read more...] about DePuy Synthes for Sale: Who Could Actually Buy the Orthopedic Giant?
Johnson & Johnson Explores Potential $20B Sale of DePuy Synthes
Johnson & Johnson is preparing a potential sale of its orthopedics unit, DePuy Synthes, in a transaction that could exceed $20 billion, according to a source cited by Reuters. The company is said to be positioning private equity firms as the most likely buyers, although it has not publicly commented on the report. Last year, J&J announced plans to separate the … [Read more...] about Johnson & Johnson Explores Potential $20B Sale of DePuy Synthes
We are proud to announce that Syntropiq will once again be a sponsor of SPINEMarketGroup in 2026
Thank you, Syntropiq! On behalf of the SPINEMarketGroup team, we sincerely appreciate your continued support as a PLATINUM sponsor for 2026.We are very proud to continue this journey together for another year! About Syntropiq We are a privately held spine interbody implant and surface technology company. Our team members with rich experience more than 25 … [Read more...] about We are proud to announce that Syntropiq will once again be a sponsor of SPINEMarketGroup in 2026
Modern Leadership: Lots of light. Little reality
Emmanuel Badet--We renamed the management. We made it up, modernized it, dressed it up in seductive words. However, behind this contemporary veneer, many organizations still operate as they did yesterday, simply with more slides, more anglicisms and much more human fatigueLet's be clear-headed. Management sometimes resembles a shadow theatre. We talk about vision, autonomy, … [Read more...] about Modern Leadership: Lots of light. Little reality
Dymicron Appoints Veteran MedTech CEO Peter Wehrly to Board of Directors
Global C-suite leader brings decades of spinal innovation and PMA experience to support Dymicron's next stage of growth OREM, Utah, Feb. 19, 2026 /PRNewswire/ -- Dymicron®, a privately held medical device company advancing next-generation spinal motion-preservation technology, today announced the appointment of Peter Wehrly, one of the spinal industry's most … [Read more...] about Dymicron Appoints Veteran MedTech CEO Peter Wehrly to Board of Directors
Carlsmed Announces first corra™ personalized Cervical Plating Procedure
The corra™ Cervical Plating System marks the debut of Carlsmed's patient-specific fixation portfolio CARLSBAD, Calif., Feb. 18, 2026 (GLOBE NEWSWIRE) -- Carlsmed, Inc. (Nasdaq: CARL) (“Carlsmed” or the “Company”), today announced the first personalized cervical plating procedure using the corra™ Cervical Plating System, the latest addition to its portfolio of personalized … [Read more...] about Carlsmed Announces first corra™ personalized Cervical Plating Procedure
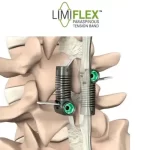
FDA Grants PMA Approval to LimiFlex(TM) Dynamic Sagittal Tether
Motion-Preserving Lumbar Spine Surgery - Stabilization Without Fusion for Patients with Degenerative Spondylolisthesis SAN CARLOS, Calif., February 18, 2026 (Newswire.com) - The U.S. Food and Drug Administration (FDA) has granted Premarket Approval (PMA) to the LimiFlex™ Dynamic Sagittal Tether, a motion-preserving system for the treatment of lumbar spinal … [Read more...] about FDA Grants PMA Approval to LimiFlex(TM) Dynamic Sagittal Tether
Spineway : 2025 annual results
Ecully, February 17, 2026, SPINEWAY 2025 annual results: * 2024 Pro forma: changes to the 2025 French General Chart of Accounts (ANC Regulation 2022-06) required the inclusion in operating income/(loss) of items that were previously recognized in exceptional income and expenses. Additionally, the Research and Innovation tax credits, which were recognized in operating … [Read more...] about Spineway : 2025 annual results
Fziomed Commemorates One Millionth Patient Treated With Its Dual-Polymer Adhesion Barrier Technology
SAN LUIS OBISPO, Calif., Feb. 18, 2026 /PRNewswire/ -- Fziomed, Inc. ("Fziomed" or the "Company"), a recognized global leader in postsurgical adhesion prevention with the best-in-class synthetic, absorbable gel technology platform, today announced that the Company has surpassed treating one million patients worldwide across its product portfolio over the … [Read more...] about Fziomed Commemorates One Millionth Patient Treated With Its Dual-Polymer Adhesion Barrier Technology
Medtronic reports strong third quarter fiscal 2026 results with highest enterprise revenue growth in 10 quarters
GALWAY, Ireland, Feb. 17, 2026 /PRNewswire/ -- Medtronic plc (NYSE: MDT), a global leader in healthcare technology, today announced financial results for its third quarter (Q3) of fiscal year 2026 (FY26), which ended January 23, 2026. Q3 Key Highlights "Q3 marks another strong quarter, delivering 6% organic revenue growth, ahead of guidance, demonstrating the strength … [Read more...] about Medtronic reports strong third quarter fiscal 2026 results with highest enterprise revenue growth in 10 quarters
Life Spine Announces MR Conditional Status for ProLift Expandable Spacer System Product Lines
HUNTLEY, Ill.--(BUSINESS WIRE)--Life Spine, a medical device company that designs, develops, manufactures, and markets products for the surgical treatment of spinal disorders, announced today its ProLift® expandable interbody portfolio has been evaluated and confirmed as MR Conditional in accordance with ASTM standards. The MR Conditional status includes ProLift, ProLift … [Read more...] about Life Spine Announces MR Conditional Status for ProLift Expandable Spacer System Product Lines
Medical Sales Isn’t One Career. Which One Are You Actually In?
Many careers in medical sales fail not because of performance, but because people are hired into the wrong type of role. From the outside, healthcare commercial roles look similar. A badge, a hospital, meetings with clinicians, and a product to represent. But inside the industry, the term “sales rep” describes fundamentally different professions. A trauma rep, a capital … [Read more...] about Medical Sales Isn’t One Career. Which One Are You Actually In?
We are proud to announce that Tsunami Medical will once again be a sponsor of SPINEMarketGroup in 2026
Thank you, Tsunami Medical! On behalf of the SPINEMarketGroup team, we sincerely appreciate your continued support as a PLATINUM sponsor for 2026.We are very proud to continue this journey together for another year! About Tsunami Medical The company has been founded in 1997 as a subcontractor of some big manufacturing companies of invasive diagnostic devices. Over … [Read more...] about We are proud to announce that Tsunami Medical will once again be a sponsor of SPINEMarketGroup in 2026
VB Spine Announces Intent to Acquire Exclusive Rights to Augmedics’ Spine Platform
NEW YORK & CHICAGO--(BUSINESS WIRE)--VB Spine LLC (“VB Spine”), the largest privately held spine company, today announced it has entered into a definitive agreement to acquire exclusive rights to the xvision Spine System® (xvision) from Augmedics, adding augmented reality (AR) navigation to VB Spine’s growing enhanced visualization portfolio. Augmedics is the first company … [Read more...] about VB Spine Announces Intent to Acquire Exclusive Rights to Augmedics’ Spine Platform
Innovation on Hold: How EPO Oppositions Affect Spine Startups — The Powehi Medical Case
Powehi Medical, a young spine startup focused on fixation systems and less rigid stability concepts, recently experienced a situation far more common in MedTech than many founders initially expect. After receiving its first European patent grant, a Swiss spine company filed an opposition at the European Patent Office (EPO). This was not a lawsuit. For a startup, it can be … [Read more...] about Innovation on Hold: How EPO Oppositions Affect Spine Startups — The Powehi Medical Case
Medtronic receives FDA clearance for Stealth AXiS™ surgical system, first integrated planning, navigation and robotics platform for spine surgery
GALWAY, Ireland, Feb. 13, 2026 /PRNewswire/ -- Medtronic (NYSE: MDT), a global leader in healthcare technology, today announced U.S. Food and Drug Administration (FDA) clearance of the Stealth AXiS™ surgical system, a next-generation platform that brings planning, navigation, and robotics together into a single, intelligent system for spine surgery. The Stealth AXiS™ system … [Read more...] about Medtronic receives FDA clearance for Stealth AXiS™ surgical system, first integrated planning, navigation and robotics platform for spine surgery
Most Spine Failures Are Predictable — The Bone Problem in Spine Surgery Nobody Budgets For
For years, spine surgery has been performed under the assumption of adequate bone quality: stiffer implants, larger corrections, more instrumented levels, and increasing use of MIS in older patients. But demographics changed before surgical philosophy did. Today, the typical degenerative case is less often a middle-aged disc herniation and increasingly an elderly patient. … [Read more...] about Most Spine Failures Are Predictable — The Bone Problem in Spine Surgery Nobody Budgets For
Vivex Announces New Peer-Reviewed Publication Demonstrating Long-Term Fusion Outcomes with VIA Form+™ in Lumbar Interbody Fusion
MIAMI, Feb. 12, 2026 /PRNewswire/ -- Vivex Biologics, Inc., a leading medical technology company developing and delivering innovative allografts for musculoskeletal and wound-care applications, today announced the publication of a new peer-reviewed study evaluating the long-term clinical and radiographic outcomes of lumbar spinal fusion procedures utilizing VIA … [Read more...] about Vivex Announces New Peer-Reviewed Publication Demonstrating Long-Term Fusion Outcomes with VIA Form+™ in Lumbar Interbody Fusion