In the past decade, the orthopedic implant industry has been quietly revolutionized through the use of additive manufacturing. Today, the penetration rate of additive production of standard-sized implants is expanding rapidly. In the future the researcher believes the majority of implants will be produced additively, creating a new revenue stream for printers and materials as […]
NEWS
Additive Manufacturing in Spine: The future is here. Why are 5 of the Leading Companies launching 3D Cages?
Additive Manufacturing (Three-dimensional printing 3DP), is a very rapidly growing industry trend, particularly in the area of spinal surgery. Given the complex anatomy of the spine and delicate nature of surrounding structures, 3DP has the potential to aid surgical planning and procedural accuracy. The technology has the potential for enhanced implant properties, as well as […]
Are you looking for a Job? This Is How You Get on a Headhunter’s Radar.50 Headhunters to Know
If you’ve made the decision that it’s time for a new job and have started to reach out to friends about possible openings. It’s nearly inevitable that you’ll soon be asked, “Have you reached out to recruiters?” Ironically, although the question seems straightforward, it’s a tough one to answer. I’ll admit, before entering the career […]
Europe spinal implants market: The Top 20 European Manufacturers Of Spinal Implants (Part III)
The European spine surgery devices market is expected to reach $3.46 billion by 2021. The spinal fusion & fixation is the largest segment in the European spine surgery devices market, with a share of 66.5% in terms of value. It is projected to grow at a CAGR of 6.2% during the forecast period. While a […]
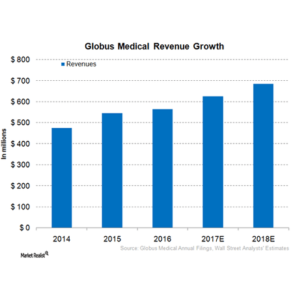
Globus Medical: Innovating Beyond The Spine Market
(SEEKINGALPHA.COM) –The orthopedic implants industry is relatively fragmented among ten large competitors who operate in the space, with some like Globus Medical (GMED), specializing in certain products such as minimally invasive spine implants. Despite Globus Medical’s recent success, it remains a relatively small player in the orthopedic implants market. Stryker (SYK), Zimmer-Biomet (ZBH), and Medtronic […]
Europe spinal implants market: The Top 20 European Manufacturers Of Spinal Implants (Part II)
The European spine surgery devices market is expected to reach $3.46 billion by 2021. The spinal fusion & fixation is the largest segment in the European spine surgery devices market, with a share of 66.5% in terms of value. It is projected to grow at a CAGR of 6.2% during the forecast period. Factors driving […]
Europe spinal implants market to reach $3.8 B by 2024.The Top 20 European Manufacturers Of Spinal Implants (Part I)
How Big is The Europe Spinal Implants Market? The Europe Spinal Implants Market is expected to exceed more than US$ 3.8 Billion by 2024 by growing at a compound annual growth rate of 5.7 percent according to a Data Bridge Market Research analysis. The German market is the greatest followed by France, United Kingdom, Italy and Spain. The major […]
This remarkable spinal implant was created by an algorithm
This coral-like form is a spinal implant. Created by Californian medical company NuVasive, it is made from titanium and fits precisely between two vertebrae. By mimicking the porousness and stiffness of human bone, it can accelerate bone growth following back surgery. Spinal surgeons typically use implants made from high-performance plastic, because the material is less […]
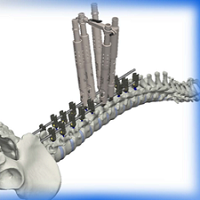
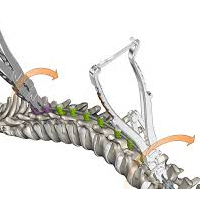
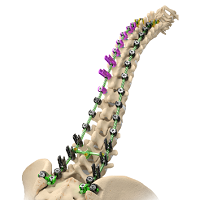
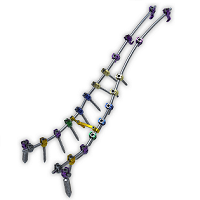
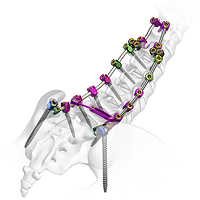
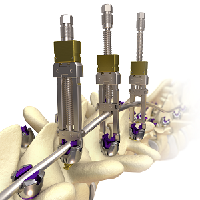
Will Spinal Deformities Drive the Global Spinal market in the next 10 years? Did you know that there are already more than 30 systems in the market?
According to Persistence Market Research, the global deformity spinal system market is driven by the increasing prevalence of spinal deformities and instabilities and spine related diseases.A rising number of spine deformity cases and increasing technological advancements will drive the global spinal implants and devices market. Deformity spinal implant keep on creating as innovation advances the […]
Black Diamond
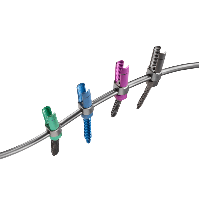
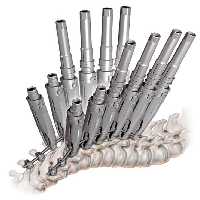
The Black Diamond Pedicle Screw System is an intuitive thoracolumbar fixation system that is ideal for use in both degenerative and deformity operations. Vibrant colors make for simplified implant identification and in-situ visualization while novel instrumentation optimizes each step of the procedure. The system’s versatile implant and instrumentation options allow for use in a minimally […]
CD HORIZON® SOLERA™ System
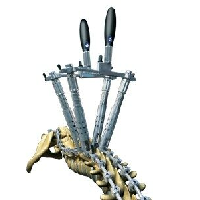
The CD HORIZON® SOLERA™ System is the first platform technology that enables surgeons to confidently and efficiently treat their patients by using: high strength, low profile multi-axial screws designed for use with multiple rod types the first-ever powered instruments being developed specifically for spinal implants minimally invasive techniques, navigation tools, and advanced reduction technology that […]
Canaveral Deformity System
The Canaveral Deformity System is designed to treat advanced complex deformity conditions. Canaveral Deformity FloSpine Brochure.pdf Features: Fast Set up Excellent Correction Adapt easily to a variety of curves Elite Locking screw drivers Screw Inserter and Headbody delivery instruments for in situ assembly Matched color coded taps to screw diameters for fast screw prep Multiple handle options […]
CD horizon® Legacy™ Spinal System
The CD Horizon® Legacy™ Spinal System can be used to treat scoliosis, a condition in which the spine develops one or more abnormal, side-to-side curves that in turn may affect the body’s overall balance and alignment, as well as possibly lead to other physical and health problems.The CD Horizon Legacy Spinal System is one of the […]
CREO® thoracolumbar stabilization system
The CREO® thoracolumbar stabilization system enhances efficiency and ease of use with intuitively designed instruments and a complete array of implant options for treating complex spinal pathologies with one system. Introducing the lowest profile 4.75 stabilization system on the market, CREO® 4.75 provides surgical solutions for small stature and pediatric patients. CREO® 5.5mm has a competitively low profile design […]
DENALI® Deformity Spinal System
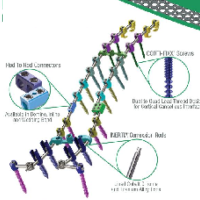
The DENALI® Deformity Spinal System is a top-loading spinal system featuring off-axis screw height adjustment and offering a complete array of screws, rod connectors, and hooks, coupled with easy-to-use instrumentation. This comprehensive system is poised to address a wide range of complex spinal pathologies. Features Complete Offering of Polyaxial Screws, Monoaxial Screws, Hooks, Rod Connectors, & […]
Daytona® System Seaspine
The Daytona® System utilizes the technology of our Malibu™ pedicle screw system to address deformity cases ranging from standard to complex. Unique implant designs, advanced materials and innovative instrumentation creates a highly flexible and intuitive system. Daytona Deformity-Seaspine.pdf Features: Extended tabs for 30mm of reduction Integrated locking cap for incremental reduction over multiple screws ESS (Enhanced […]
EVEREST® Deformity Spinal System
The EVEREST® Deformity Spinal System features a top-loading pedicle screw in a variety of screw types and the ability to accommodate titanium and cobalt chrome rods of two different diameters. The revolutionary instrumentation is designed to address the most difficult correction maneuvers for complex spinal pathologies. EVEREST VIDEO ANIMATION EVEREST Minimally Invasive.SGT-K2M-Stryker.pdf Features: Dual-lead Thread Pattern for […]
Excella III-D® Spinal Deformity System
The Innovasis Excella III-D system is the perfect combination of practicality and efficiency. Through ingenuitive instrumentation that isn’t overly complicated, we make complicating surgery easier. The Excella®-M Spinal System consists of 6AL-4V Titanium alloy implants meant to be used in a system. The monoaxial bone screws are offered in a variety of different lengths ranging from […]
EXPEDIUM Spine System
The EXPEDIUM Spine System is a innovative spine solution with technological advancements that truly differentiate it from other systems available. The EXPEDIUM Spine System is a rod based platform. The system consists of the following: • Polyaxial Screws (Single Innie, Dual Innie, Favored Angle Screw) • Monoaxial Screws • Extended Tab Implants • Hooks • […]
Firebird Deformity
The Firebird Deformity Correction System offers even greater options for patients with spinal deformity. When surgically treating a variety of thoracolumbar and sacral pathologies, the Firebird Deformity Correction System offers additional implant and instrument options needed to perform complex spine procedures. Firebird VIDEO ANIMATION Firebird.Brochure-Orthofix.pdf Firebird Deformity-Orthofix.pdf Benefits: Implants: CoCr rods for stiffness and strength Closed […]
ILIAD Spinal Fixation System
ILIAD Spinal Fixation System provides simple and comprehensive stabilization solutions for spinal fixation. The Patented Reverse DOVETAIL Locking System with LINEAR SLOT is a foundation of the ILIADTM system. This unique thread design practically eliminates cross threading, prevents splaying of the screw head and increase the holding moment up to 30Nm. ILIAD Spine System VIDEO […]
INERTIA Deformity Correxxion System
The INERTIA® Deformity Correxxion System, an extension to the Inertia Pedicle Screw System, was designed to address a greater range of complex spinal pathologies from T1 to the Pelvis. INERTIA Deformity Correxxion System VIDEO ANIMATION Inertia Deformity.STG-Nexxt Spine.pdf Inertia Deformity Correxxion System provides the addition of Colbolt Chrome Rods, Uniplanar and Reduction Screws, Offset Connectors, adjustable […]
Invictus™
Invictus is ATEC’s comprehensive spinal fixation platform, designed to treat a range of pathologies with intraoperative adaptability and surgical predictability through an open, MIS, or hybrid approach. Key Features DEPENDABLE: Leverages patented and dependable helical flange tulip technology to reduce the potential to cross-thread and help eliminate tulip splay. ADAPTABLE: Adapts intraoperatively to surgical techniques with robust […]
LANCER™
The ChoiceSpine LANCER™ Open Pedicle Screw System is a posterior spinal fixation system consisting of various polyaxial screws, rods, cross-connectors, and hooks to accommodate various spinal anatomies. LANCER™ is intended to provide immobilization and stabilization of spinal segments in skeletally mature patients as an adjunct to fusion for degenerative disc disease, spondylolisthesis, trauma, spinal stenosis, […]
Mercury Total Spine
Mercury is the Deformity and Iliac fixation system from Spinal Elements. Mercury System VIDEO ANIMATION Mercury Total Spine.Brochure-Spinal Elements.pdf Features: Multiple screw head options Screw sizes to match any patient anatomy Various rod connectors for surgical versatility About Spinal Deformity Scoliosis is an abnormal curvature of the spine that can occur in any age group. Scoliosis […]
MESA® 2 Deformity Spinal System
The next-generation Mesa 2 screws are top-loading, low-profile, and feature Zero-Torque Technology . The streamlined instrumentation is designed for efficiency and ease of use. This system is poised to address the most difficult correction maneuvers for complex spinal pathologies. MESA 2 Deformity VIDEO ANIMATION Features: Dual-lead Thread for Screw Faster Insertion Zero-Torque Technology® No Profile […]
mont blanc 3D+
The mont blanc 3D+ is a system of implants and instruments for the correction of idiopathic scoliosis by posterior approach.Its is an Implant system for vertebral column inner fixation at thoracolumbar level by posterior approach open surgery, devised for global direct correction of severe spine deformities. mont blanc 3D+ VIDEO ANIMATION mont blanc 3D-SGT.Spineway.pdf Benefits: […]