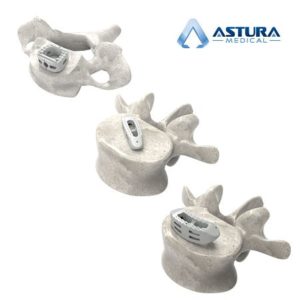
IRVING, TX – November 9, 2020 – Astura Medical, a high-growth, innovative spine technology company, today announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Dolomite Stand-Alone Anterior Cervical Stabilization System. Expanding on the rapid adoption and recent success of the company’s other integrated plate and spacer technologies […]
FDA
INDIUS Medical Technologies is proud to announce the US FDA 510K clearance of the ACURA Thoracolumbar Posterior Stabilization System
Indius Medical Technologies, an Indo – US multinational Startup focused on the development of US FDA compliant global quality products for the treatment of spinal disorders has FDA clearance for the ACURA Thoracolumbar Posterior System. The ACURA System features the following implants: Paediatric Screw Head Profile with Adult Load Bearing Strength enables O.R. Efficiency Anti-Splay […]
Nvision Biomedical Technologies: First FDA clearance for an osteotomy wedge system made of PEEK-OPTIMA™ HA Enhanced
San Antonio, Texas/ Thornton Cleveleys (UK), October 28, 2020 – Nvision Biomedical Technologies, a San Antonio-based medical device and implant manufacturer, has FDA clearance for the first osteotomy wedge system made from PEEK-OPTIMA HA Enhanced, a polymer from Invibio Biomaterial Solutions (‘Invibio’). The Trigon® Stand-Alone Osteotomy Wedge Fixation System has osteoconductive properties that promote multi-directional bone healing and […]
Implanet and SeaSpine announce FDA clearance of the Mariner Cap System
Bordeaux, Boston, October 19, 2020 – 5.45 pm CEST: IMPLANET (Euronext Growth: ALIMP, FR0013470168, eligible for PEA-PME equity savings plans), a medical technology company specializing in vertebral and knee-surgery implants, and SeaSpine Holdings Corporation (NASDAQ: SPNE) today announce the clearance of the Mariner Cap System in the United States, which combines SeaSpine’s Mariner® pedicle screw system with […]
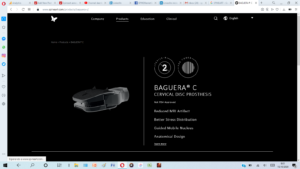
BAGUERA® C in the US – Investigational Device Exemption (IDE) FDA Approval 2 Levels
Following the recent FDA approval to initiate a pivotal IDE clinical study for the BAGUERA® C artificial cervical disc at 1-level, Spineart is proud to announce that it has received approval from the FDA to initiate as well a pivotal IDE clinical study for BAGUERA® C at two contiguous levels.Both studies will be randomized controlled trials comparing the […]
SMAIO announces the FDA 510k approval of the KHEIRON® spinal fixation system
SMAIO is delighted to share that our spinal fixation system KHEIRON® just received FDA-510(k) clearance.This big step for SMAIO will allow us to start commercializing our innovative solutions on the US market and will sustain our international development. KHEIRON is a Posterior thoraco-lumbar fixation system offering a full range of implants to adress the main […]
NeuroStructures announces the FDA 510k approval of the Oculus™-SA Lumbar Cage System
NeuroStructures is excited and pleased to announce the FDA 510k approval of the Oculus™-SA Lumbar Cage System, which features our “Neuro-Lock” surface technology to enhance fusion. The Oculus™-SA allows the surgeon to use multiple integrated screw construct options for any surgeons’ preference. Along with a zero profile design and locking plate for secure visual confirmation, […]
IZI Medical Receives CE Mark Approval for Kiva™ VCF Treatment System
OWINGS MILLS, Md., Sept. 10, 2020 /PRNewswire/ — IZI Medical Products, LLC (“IZI”), a leading manufacturer of interventional radiology devices, announces that it has received CE Mark approval in Europe for the KivaTM Vertebral Compression Fracture (VCF) Treatment System. Kiva is a unipedicular PEEK implant-based treatment solution for VCFs that has seen clinical and commercial success in the US. “We […]
Life Spine Announces FDA 510(k) Clearance for the for the PLATEAU®-A TI Anterior Lumbar Spacer System
Huntley, IL, August 5 , 2020 –Life Spine, a medical device company that designs, develops, manufactures and markets products for the surgical treatment of spinal disorders, announced today that it has received clearance from the U.S. Food & Drug Administration (FDA) to market the PLATEAU-A Ti Anterior Lumbar Spacer System. “With the increased usage of […]
NeuroStructures is pleased to announce the FDA 510k approval of the Transept™ Cervical Plating System
NeuroStructures is excited and pleased to announce the FDA 510k approval of the Transept™ Cervical Plating System which features the ability to achieve extreme screw angulation (up to 38°) and extremely short plates (10mm). The plate has an ultra-thin profile and comes with a wide spectrum of fixed, variable, self-tapping, and self-drilling screws. Also included, […]
icotec ag Receives Approvals for Two KONG® VBR Spinal Systems Made with BlackArmor® and Ti-iT® in Europe and the US
ALTSTAETTEN, Switzerland, June 18, 2020 /PRNewswire/ — icotec ag announces that the KONG®-TL and the KONG®-C vertebral body replacement systems with the unique Titaniumcoating (Ti-iT®) receive FDA 510(k) clearance in the United States and CE approval in Europe .”This is exciting news for icotec ag and our team as it allows us to expand our portfolio in multiple countries simultaneously, to include implants […]
Meditech Spine Receives FDA Clearance for its CURE™ OPEL-L (S) system
ATLANTA, June 1, 2020 /PRNewswire/ — Meditech Spine has received FDA 510(k) clearance to market the CURE™ Opel-L (S) system, a new lumbar plate option which expands upon the previously cleared CURE™ LP Plate System and compliments its Talos®-A (HA) Interbody system. With this approval, Meditech will now offer an Interbody/Plate assembly for the anterior lumbar spine. By […]
SpineEX Receives Additional FDA Clearance for Innovative Sagittae Lateral Lumbar Interbody Fusion System
FREMONT, Calif., March 25, 2019 (GLOBE NEWSWIRE) — SpineEX, Inc., a medical device company focused on the design, development and marketing of products for spine disorders, received an additional U.S. Food and Drug Administration (FDA) clearance for its innovative Sagittae Lateral Lumbar Interbody (LLIF) Fusion System. This additional 510(k) clearance will enhance SpineEX’s ability to […]
Implanet: FDA Clearance for JAZZ Cap® System
BORDEAUX, France & BOSTON: IMPLANET (Paris:ALIMP) (OTCQX:IMPZY) (Euronext Growth: ALIMP, FR0010458729, eligible for PEA-PME equity savings plans), a medical technology company specializing in vertebral and knee surgery implants, has announced that it has received 510(k) authorization from the Food and Drug Administration (FDA) for its JAZZ Cap® System, designed to meet the constraints of vertebral […]
Tenon Medical, Inc. Receives FDA Clearance For Sacroiliac Joint Fusion System
SAN RAMON, Calif., June 20, 2018 /PRNewswire/ — Tenon Medical, Inc., a manufacturer of minimally invasive instruments and implants for sacroiliac joint fusion surgery, today announced that it has received 510(k) clearance from the United States Food and Drug Administration (FDA) for the Catamaran™ Sacroiliac Joint Fixation System (CAT SIJ Fixation System) specifically indicated for sacroiliac joint fusion […]
Centinel Spine Announces 510(k) Clearance of FLX™ Platform of 3D Printed All-Titanium Interbodies
NEW YORK, June 7, 2018 /PRNewswire/ — Centinel Spine, LLC is pleased to announce that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its FLX™ Platform of Integrated Interbody™ and non-integrated interbody fusion devices. Centinel Spine is the largest privately-held spine company, focused on anterior column reconstruction. FLX devices are 3D-printed, […]
Astura Medical Receives FDA 510(k) Clearance For Cervical and Lumbar HA PEEK Interbody Systems
CARLSBAD, CA – June 4, 2018 – Astura Medical, a high growth, innovative spine technology company, today announced that it received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its ALTA ACDF Interbody Spacers and HALF DOME Lumbar Interbody Spacers in PEEK-OPTIMAä HA Enhanced from Invibio Biomaterial Solutions. Combining the clinically proven […]
Spineology Rampart One™ Standard ALIF Interbody Fusion System Gains FDA Clearance for Stand-Alone Use
ST. PAUL, Minn.–(BUSINESS WIRE)–Spineology Inc., announces FDA clearance of its Rampart One™ Standard ALIF device allowing it to be used with or without supplemental fixation. “Following the success of our beta launch, this stand-alone clearance will provide additional momentum as we prepare for the full market release of the Rampart One ALIF Interbody Fusion System,” […]
Camber Spine Announces 510(k) Approval Of ENZA™-A Titanium ALIF
WAYNE, Pa., June 4, 2018 /PRNewswire/ — Camber Spine today announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its ENZA™-A Titanium Anterior Lumbar Interbody Fusion (ALIF) system, a unique, minimally invasive interbody fusion device providing integrated fixation. Daniel Pontecorvo, CEO, said, “ENZA™-A leverages two of Camber […]
Why an FDA Panel Shot Down Intrinsic’s Spinal Implant?
(MDDIONLINE.COM) –Although FDA doesn’t have to follow the recommendation of its advisory committees, a negative panel vote is never a good sign for a company hoping to bring a new device to the market. Intrinsic Therapeutic learned the hard way Tuesday that it’s usually best if clinical trials follow the protocol that was initially communicated to […]
ChoiceSpine™ Granted FDA Clearance for 3D Printed Vertebral Body Replacement Device
KNOXVILLE, TN (PRWEB) OCTOBER 04, 2017-ChoiceSpine LP, a privately-held spinal device manufacturer based in Knoxville, TN, announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market HAWKEYE Ti, a 3D Printed Titanium Vertebral Body Replacement (VBR) device. “Additive manufacturing techniques create intricate three-dimensional implants, layer by layer, allowing a […]
Nexxt Spine Announces FDA Clearance for the NEXXT MATRIXX™ System
NOBLESVILLE, Ind.–(BUSINESS WIRE)–Nexxt Spine, LLC, a medical device company focused on designing, manufacturing, and distributing innovative spinal solutions, today announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market the NEXXT MATRIXX™ System. The NEXXT MATRIXX™ System of 3D printed porous titanium leverages Nexxt generation technology to create […]
Tyber Medical Expands FDA 510(k) Clearance Of Headed And Snap-Off Screws
MORRISTOWN, N.J., June 14, 2016 /PRNewswire/ — Tyber Medical, LLC, a company focused on providing rapid private label commercialization with innovative orthopedic and spine devices including bioengineered surfaces, announces FDA 510K clearance of their headed and snap-off screws systems. Tyber Medical’s private label partners now have access to the most extensive range of trauma screws […]
ChoiceSpine Titanium Interbody 510(k)
KNOXVILLE, TN, June 7, 2015 – ChoiceSpine, a Knoxville, TN based spinal implant company, today announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market titanium lumbar interbody fusion devices. ChoiceSpine plans to offer titanium interbody devices for traditional PLIF (Posterior Lumbar Interbody Fusion) and TLIF (Transforaminal Lumbar Interbody […]
Wenzel Spine, Inc. Announces FDA Clearance to Launch VariLift
AUSTIN, Texas–(BUSINESS WIRE)–Wenzel Spine, Inc., a pioneer medical device company focused on providing minimally invasive, stand-alone alternatives to traditional spinal fusion, announced today that it received clearance from the U.S. Food and Drug Administration (FDA) to market VariLift®-LX as an interbody fusion device for stand-alone use in the lumbar spine. VariLift®-LX represents the next generation […]
Xtant™ Medical Receives Clearance of Expanded Indication for Ilium Fixation and Deformity
BELGRADE, Mont., Dec. 01, 2015 (GLOBE NEWSWIRE) — Xtant Medical Holdings, Inc. (NYSE:XTNT), a leader in the development of regenerative medicine products and medical devices, today announced that its wholly owned subsidiary, X-spine Systems, Inc. received FDA clearance of expanded indications to include ilium fixation as well as extended length screws for the Fortex® and […]
Xtant™ Medical Receives Clearance of Expanded Indication for Ilium Fixation and Deformity
BELGRADE, Mont., Dec. 01, 2015 (GLOBE NEWSWIRE) — Xtant Medical Holdings, Inc. (NYSE:XTNT), a leader in the development of regenerative medicine products and medical devices, today announced that its wholly owned subsidiary, X-spine Systems, Inc. received FDA clearance of expanded indications to include ilium fixation as well as extended length screws for the Fortex® and […]