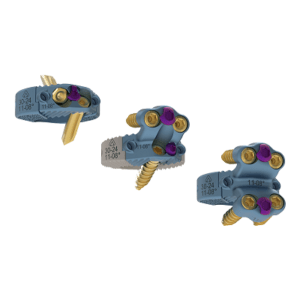
PALM BEACH GARDENS, Fla., May 12, 2022 (GLOBE NEWSWIRE) — Accelus, a privately held medical technology company focused on accelerating the adoption of minimally invasive surgery (MIS) as the standard of care in spine, today announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its FlareHawk TiHawk™11 Interbody […]
FDA
510(k) Clearance received for Anterior Cervical Plate System Aggeris-C.
Clariance Spine is proud to announce that Aggeris-C received 510(k) clearance. Aggeris-C is low profile anterior cervical plate system designed with the following features in mind: PRE-LORDOSED PLATEPre-lordosed plate conforms to the patient’s cervical vertebrae for enhanced stability. LOW PROFILELow profile to reduce the risk of contact with and irritation to soft tissues. SECURED PLATEVariable angle […]
Osseus Announces FDA 510(k) Clearance of the Pisces-SA Standalone ALIF Interbody System
DALLAS, TX, May 9, 2022 – Osseus, an innovative spinal solutions company, announces FDA 510(k) clearance and launch of the Pisces™-SA Standalone ALIF Interbody System. The Pisces™-SA interbody can be used with both bone screws and alternative fixation bone anchors allowing for increased intraoperative flexibility. Biomechanical testing proved that the Pisces™-SA anchors provide better expulsion resistance than the competition and perform comparably with traditional screw-based standalone ALIF constructs in stabilizing injured spinal segments. The Pisces™-SA is the first of its […]
Astura Medical Receives FDA 510(k) Clearance For El Capitan Oblique Anterior Lumbar Interbody Fusion System
IRVING, TX – April 4, 2022 – Astura Medical, a high-growth, innovative spine technology company, today announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its El Capitan Oblique Anterior Lumbar Interbody Fusion (ALIF) System. Built from the foundation of success with the company’s other integrated plate and […]
SpineGuard obtains FDA clearance for commercial release of its “Threaded PediGuard” in anterior approach spine surgery
PARIS and BOULDER (CO), April 4, 2022, at 06:00pm CEST – SpineGuard (FR0011464452 – ALSGD) (Paris:ALSGD), an innovative company that deploys its DSG (Dynamic Surgical Guidance) sensing technology to secure and streamline the placement of bone implants, announced today the authorization under 510K #220160 by the FDA (Food and Drug Administration) to commercialize in the United […]
Life Spine Announces Additional 510k Clearance for SImpact Si Joint Fixation System
Huntley, IL, March 29th, 2022 –Life Spine, a medical device company that designs, develops, manufactures and markets products for the surgical treatment of spinal disorders, announced today that is has received clearance from the U.S. Food & Drug Administration (FDA) to market SImpact Si Joint Fixation System for Posterior-Oblique approaches. Whether the physician is performing […]
SpineUp Announces U.S. FDA 510(K) Clearance for its New Cervical Cage in Peek HA-Enhanced
February 28, 2022– SpineUp a privately held medical technology company has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its brand new cervical cage in Peek Optima HA-Enhanced, Romero.They offer to the surgeons 4 types of cervical cages: Lordotic with screw (self-tapping or self-drilling), anatomic with screw, anatomic and lordotic without […]
VySpine Announces FDA Clearance for ClariVy Cervical IBF System
Tallahassee, FL – February 1, 2021—VySpine, VySpine, a spine innovation leader using differentiated materials and designs, announced today that it has received 510(k) clearance from the FDA for the its ClariVy Cervical IBF System which is designed for use in anterior cervical discectomy with fusion (ACDF) procedures. The ClariVy Cervical IBF System features cervical interbody fusion devices made […]
VUZE Medical Announces U.S. FDA 510(K) Clearance for its VUZE System
RA’ANANA, Israel – January 18, 2022 – VUZE Medical, a privately held medical technology company aimed at transforming image guidance and verification in minimally invasive spine surgeries, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its VUZEÔ System. Using proprietary image processing, the VUZE System is a software-only solution that overlays a graphical […]
Spinal Elements® Announces FDA 510(k) Clearance of Lucent® 3D Additive Manufactured Interbody Devices
Carlsbad, CA – April 20, 2021 – Spinal Elements, a spine technology company, today announced the first 510(k) clearance in the Lucent® 3D line of 3D-printed interbody devices. In developing Lucent 3D, Spinal Elements has taken advantage of the unique capabilities of 3D printing by printing a functionally unique multi-component device in a single printing […]
NuVasive’s Simplify Disc Receives FDA Approval for Two-Level Cervical Total Disc Replacement
SAN DIEGO, April 6, 2021 /PRNewswire/ — NuVasive, Inc. (NASDAQ: NUVA), the leader in spine technology innovation, focused on transforming spine surgery with minimally disruptive, procedurally integrated solutions, today announced the NuVasive Simplify® Cervical Artificial Disc (Simplify Disc) received approval from the U.S. Food and Drug Administration (FDA) for two-level cervical total disc replacement (cTDR). “This approval is an incredible […]
Premia Spine Announces FDA Breakthrough Device Designation for Its TOPS™ Spinal Arthroplasty System
NORWALK, Conn. – March31, 2021 – Premia Spine, a medical technology company aiming to change the way debilitating chronic leg and back pain is treated, today announced that its TOPS facet arthroplasty system won Breakthrough Device Designation from the U.S. Food & Drug Administration. TOPS is the first and only facet joint replacement system for […]
NGMedical GmbH Receives FDA Clearance for Its BEE® HA Cervical Cage System Made From PEEK-OPTIMATM HA Enhanced
SCOTTSDALE, Ariz., March 30, 2021 /PRNewswire/ — NGMedical, a German medical device manufacturer exclusively focused on creating innovative technologies for spinal application, announces FDA clearance for its anatomically shaped cervical interbody BEE® HA, made of the innovative PEEK-OPTIMA™ HA Enhanced from Invibio Biomaterial Solutions, a global leader in high-performance biomaterial solutions. BEE® HA was developed to optimize fusion while maintaining […]
Aurora Spine Receives FDA 510(K) Clearance for Its Proprietary APOLLO Anterior Cervical Plate
CARLSBAD, Calif., March 25, 2021 (GLOBE NEWSWIRE) — Aurora Spine Corporation (“Aurora Spine” or the “Company”) (TSXV: ASG) (OTCQB: ASAPF), a designer and manufacturer of innovative medical devices that improve spinal surgery outcomes, announced today that it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its proprietary APOLLO Anterior Cervical Plate (ACP) […]
Life Spine Announces FDA 510(k) Clearance for the PROLIFT® Wedge Expandable Spacer System
Huntley, IL, March 18th, 2021 –Life Spine, a leading medical device company that designs, develops, manufactures and markets products for the surgical treatment of spinal disorders, announced today that it has received clearance from the U.S. Food & Drug Administration (FDA) to market the PROLIFT Wedge Expandable Spacer System. The PROLIFT Wedge Expandable Interbody Spacer […]
4WEB Medical Receives 510(k) Clearance for its Lumbar Plating Solution
DALLAS, March 17, 2021 /PRNewswire/ — 4WEB Medical, an orthopedic device company focused on developing innovative implants with an Advanced Structural Design utilizing its proprietary Truss Implant Technology™, announced today that the company has received 510(k) clearance from the U.S. Food & Drug Administration to market its Lumbar Plating Solution (LSTS-PS). The new plating solution consists of a […]
Fusion Robotics™ Receives 510(k) Clearance for Spinal Navigation & Robotics System
BOULDER, Colo., Feb. 25, 2021 /PRNewswire/ — Fusion Robotics LLC, a spinal robotics and navigation company today announced receiving 510(k) clearance to market their 3D imaging compatible navigation and robotic targeting system for spine surgery in the U.S. market. The Fusion Robotics System addresses the key limitations of current spinal navigation and robotics systems by offering greater […]
Brainlab Loop-X Mobile Imaging Robot and Cirq Robotic Alignment Module for Spine Both Receive FDA clearance
Chicago, February 22, 2021—Brainlab announced today that the company has reached two major milestones with the FDA clearance for both Loop-X® Mobile Imaging Robot and Cirq®, a robotic surgical system. Following on CE mark approvals last summer, the FDA clearance paves the way for Brainlab to now enter the US markets with the Cirq robotic […]
Precision Spine® Receives FDA 510(K) Clearance for the Dakota ACDF™ Standalone System
February 4, 2021 – Parsippany, NJ – Precision Spine, Inc., a medical device company dedicated to Madein-the-USA manufacturing, announced today that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the Dakota ACDF™ Standalone System in the treatment of degenerative disc disease (DDD). The Dakota ACDF system features a titanium […]
NGMedical GmbH Receives FDA Clearance for Its AM Titanium Cervical Interbody BEE®
SCOTTSDALE, Ariz., Feb. 3, 2021 /PRNewswire/ — NGMedical, a German medical device manufacturer exclusively focused on creating innovative technologies for spinal application, announces FDA clearance for its purely additively manufactured titanium cervical BEE® cage. The BEE® cage is designed to challenge the limits of additive manufacturing. The purposefully designed honeycomb endplate design reduces the risk of subsidence while allowing […]
Spinal Elements® Announces FDA Clearance of Lucent® XPCurved Expandable TLIF Device
Carlsbad, CA – January 26, 2021 –Spinal Elements, a spine technology company, today announced the FDA clearance of the Lucent® XP-Curved Expandable Interbody Device. Lucent XP-Curved is the third platform in the Lucent XP family of expandable devices and the fifth expandable device within Spinal Elements’ MIS Ultra™ suite of products and procedures. The device and […]
Inspired Spine announces Trident™ SI Joint Screw System Secures FDA 510(k) Approval
BURNSVILLE, Minn., Jan. 26, 2021 /PRNewswire/ — Inspired Spine has announced the Trident SI Joint Screw System, manufactured by Advanced Research Medical (ARM), has secured FDA 510(k) marketing clearance. “We continue to raise the standard of care for back pain treatment as this SI Joint fusion technique represents another Inspired Spine breakthrough for surgeons and patients alike,” stated […]
ChoiceSpine™ Announces Line Extension of Tiger Shark
KNOXVILLE, Tenn.–(BUSINESS WIRE)–ChoiceSpine LLC, a privately held spinal device manufacturer based in Knoxville, TN, announced today that the Food and Drug Administration (FDA) has provided additional 510(k) clearance to the Tiger Shark Cervical Spacer System. Tiger Shark C is comprised of titanium alloy and constructed into an anatomical form, complementing the shape of vertebral bodies. […]
Medtronic Strengthens Leadership in Navigated, Robotic Spine Surgery with FDA Clearance of Midas Rex™ Drills and Navigated Disc Prep and Interbodies for U.S. with Mazor™ Robotic Guidance System
DUBLIN, Dec. 16, 2020 /PRNewswire/ — Medtronic plc (NYSE: MDT) announced that the U.S. Food and Drug Administration (FDA) has cleared the use of navigated interbody and Midas Rex™ high speed drills with the Mazor™ Robotic Guidance System earlier than originally anticipated. The Mazor™ platform now provides surgeons with unprecedented procedural integration by seamlessly combining the power of Midas Rex™ drills […]
SeaSpine Announces FDA 510(k) Clearance for Amended Indication for NanoMetalene® Surface Technology
CARLSBAD, Calif., Dec. 15, 2020 (GLOBE NEWSWIRE) — SeaSpine Holdings Corporation (NASDAQ: SPNE), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its NanoMetalene® surface technology with amended indications. NanoMetalene describes […]
icotec ag Receives FDA Clearance for the VADER® Pedicle – System with Bone Cement Augmentation
ALTSTAETTEN, Switzerland, Nov. 10, 2020 /PRNewswire/ — icotec ag, the leading medical device manufacture of Carbon/PEEK spinal implants, announces the U.S. Food and Drug Administration (FDA) 510(k) clearance to market the VADER® Pedicle – System for use with bone cement for augmentation or without. The VADER® Pedicle System is intended to restore the integrity of the spinal column even […]
NuVasive’s Thoracolumbar Interbody Portfolio Receives First Clearance in the US for the Treatment of Sagittal Deformities
SAN DIEGO, Nov. 4, 2020 /PRNewswire/ — NuVasive, Inc. (NASDAQ: NUVA), the leader in spine technology innovation, focused on transforming spine surgery with minimally disruptive, procedurally integrated solutions, today announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its thoracolumbar interbody portfolio to include the treatment of multi-level sagittal deformities of the […]