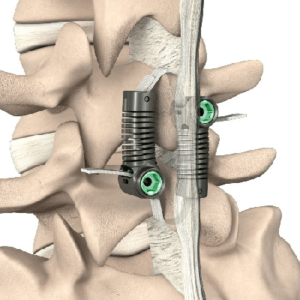
IRVING, TX – February 16, 2024 –Astura Medical, a leader in innovative spinal surgery technologies, is proud to announce that it has received FDA 510(k) clearance for its Dominion Expandable Corpectomy System. Designed with patient outcomes in mind, the Dominion Expandable Corpectomy System features state-of-the-art, expandable columns with modular adjustable endplates that allow for a […]
FDA
SurGenTec® Receives FDA Clearance for OsteoFlo® HydroPutty™, a Hydrophilic Synthetic Bone Graft
BOCA RATON, Fla.–(BUSINESS WIRE)–SurGenTec, a pioneering medical device company specializing in orthopedic and spine technologies, proudly announces the first implantations and 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its revolutionary OsteoFlo HydroPutty Synthetic Bone Graft. OsteoFlo HydroPutty represents a significant advancement in bone graft technology, featuring a combination of proprietary hydrophilic […]
Nevro Receives FDA 510(k) Clearance to Use SI Fixation System Without Need to Include Lateral Screw
REDWOOD CITY, Calif., Feb. 28, 2024 /PRNewswire/ — Nevro Corp. (NYSE: NVRO), a global medical device company delivering comprehensive, life-changing solutions for the treatment of chronic pain, today announced that the U.S. Food and Drug Administration (FDA) cleared its sacroiliac joint fusion device, which will be marketed as Nevro1, without the need to include the screw (“NevroFix™”). Nevro1, […]
SI-BONE, Inc. Receives FDA 510(k) Clearance for a Smaller Diameter iFuse Bedrock Granite Implant with an Expanded Indication and Application
SANTA CLARA, Calif., Jan. 30, 2024 (GLOBE NEWSWIRE) — SI-BONE, Inc., (Nasdaq: SIBN), a Silicon Valley-based medical device company dedicated to solving musculoskeletal disorders of the sacropelvic anatomy, announces FDA 510(k) premarket clearance of the iFuse Bedrock Granite® Implant System (Granite) in a smaller (9.5 mm) diameter with both an expanded indication in pediatric patients […]
Inspire® 3D Printed Trabecular PEEK™ With HAFUSE® Lumbar Interbody System Cleared by FDA
HUNTSVILLE, Ala., January 22, 2024 (Newswire.com) – Huntsville, AL based technology manufacturer Curiteva announced FDA 510(k) clearance of the Inspire 3D Printed Trabecular PEEK Lumbar Interbody Fusion System for use in anterior, transforaminal, posterior, and lateral lumbar interbody fusion procedures. This marks the company’s second FDA cleared 3D printed PEEK implant with HAFUSE, following the approval […]
Providence Medical Technology Announces FDA Clearance of CAVUX® FFS-LX Lumbar Facet Fixation System for 1- and 2-Level Lumbar Spinal Fusion
PLEASANTON, Calif., Jan. 10, 2024 /PRNewswire/ — Providence Medical Technology, Inc. (PMT), a medical device innovator focused on improving surgical outcomes for high-risk spine surgery patients, announced that the U.S. Food and Drug Administration (FDA) has cleared its CAVUX® FFS-LX: Lumbar Facet Fixation System for use in lumbar spinal fusion surgery. CAVUX® FFS-LX is a novel integrated cage and screw […]
SURGLASSES received FDA 510(k) Clearance for Caduceus S
TAICHUNG, Taiwan, Dec. 19, 2022 /PRNewswire/ — SURGLASSES announced today that Caduceus S AR Spine Navigation System has received 510(k) clearance from the U.S. Food and Drug Administration and is ready to launch its products into the US market in the first quarter of 2023. Caduceus S is an augmented reality spine navigation system that will require only few-shots […]
Empirical Spine Completes Full Premarket Approval (PMA) Submission to FDA for LimiFlex™ Dynamic Sagittal Tether™
SAN CARLOS, Calif., Dec. 19, 2022 /PRNewswire/ — Empirical Spine, Inc., a medical device company creating a new class of spinal implant, recently completed the final step in the U.S. Food & Drug Administration (FDA) submission process for the LimiFlex™ Dynamic Sagittal Tether™ (DST). The Premarket Approval (PMA) submission included Module III, with data and analysis of the two-year […]
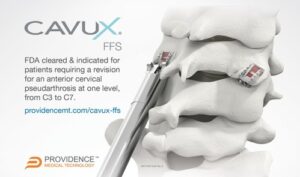
Providence Medical Technology announces FDA Clearance of the CAVUX® Facet Fixation System for the treatment of Cervical Pseudarthrosis
PLEASANTON, Calif., Dec. 15, 2022 /PRNewswire/ — Providence Medical Technology, Inc., a medical device innovator focused on improving surgical outcomes for high-risk spine surgery patients, announced that the U.S. Food and Drug Administration (FDA) has cleared its CAVUX® Facet Fixation System (FFS) for a new indication. The Facet Fixation System (FFS) is a novel integrated cage and screw […]
Life Spine Announces FDA 510(K) Clearance for the TruLift® Lateral Expandable Spacer System and Lateral Plate System
Huntley, IL, December 14th, 2022–Life Spine, a medical device company that designs, develops, manufactures and markets products for the surgical treatment of spinal disorders, announced today that it has received clearance from the U.S. Food & Drug Administration (FDA) to market the TruLift Lateral Expandable Spacer System and Lateral Plate System. Utilizing expandable technology and […]
Eminent Spine’s ALIF Stand-Alone System Receives FDA 510(K) Clearance in October 2022
Plano, TX, November 08, 2022 –(PR.com)– Eminent Spine received 510(K) approval of their ALIF Stand-Alone System as of October 17, 2022. Eminent Spine will showcase the ALIF Stand-Alone System at the 50th CSRS conference in San Diego, CA on November 16 – 19, 2022. The ALIF implant was designed with the following: a tapered nose which […]
Accelus Announces FDA 510(k) Clearance for Remi Robotic Navigation System Software Update that Allows Image Acquisition with all Major 3D Imaging Systems
PALM BEACH GARDENS, Fla., Nov. 03, 2022 (GLOBE NEWSWIRE) — Accelus, a privately held medical technology company focused on accelerating the adoption of minimally invasive surgery (MIS) as the standard of care in spine, today announced that the U.S. Food & Drug Administration (FDA) had cleared Accelus’s 510(k) application for its Remi™ Robotic Navigation System […]
ulrich Medical USA™ Receives 510(k) Clearance for Flux-C™ 3D Printed Porous Titanium Cervical Interbody Prior to NASS
ST. LOUIS, Oct. 13, 2022 /PRNewswire/ — ulrich medical USA, Inc., a privately held medical device company focused on developing and commercializing musculoskeletal implant technologies in the United States, today announced the FDA has given 510(k) clearance of its Flux-C 3D printed porous titanium cervical interbody device. “Surgeons have many options for cervical interbodies. The Flux-C porous titanium device […]
CTL Amedica gains FDA clearance for MONDRIAN™ ALIF Cage System with Supplementary Fixation Plate
DALLAS (PRWEB) OCTOBER 11, 2022–CTL Amedica Corporation has received official 510k clearance from the U.S. Food and Drug Administration (FDA) to market its MONDRIAN™ Anterior Lumbar Interbody Fusion (ALIF) Cage System with Supplementary Fixation Plate. The MONDRIAN™ ALIF Cage System with Supplementary Fixation Plate, designated K213641/S003, is an integrated plate-and-cage construct, that can be manufactured in […]
Life Spine Announces FDA 510(k) Clearance for the GHOST® 3D-Printed Titanium Spacer System
HUNTLEY, Ill.–(BUSINESS WIRE)–Life Spine, a leading medical device company that designs, develops, manufactures and markets products for the surgical treatment of spinal disorders, announced today that it has received clearance from the U.S. Food & Drug Administration (FDA) to market the GHOST 3D-Printed Titanium Spacer Systems. GHOST 3D-Printed Titanium Spacer System expands on Life Spine’s […]
Aurora Spine Corporation Announces FDA 510(k) Clearance for its SiLO TFX™ MIS Sacroiliac Joint Fixation System
CARLSBAD, Calif., Oct. 04, 2022 (GLOBE NEWSWIRE) — Aurora Spine Corporation (“Aurora Spine” or the “Company”) (TSXV: ASG) (OTCQB: ASAPF), a designer and manufacturer of innovative medical devices that improve spinal surgery outcomes, today announced it has received 510(k) clearance from the United States Food and Drug Administration (FDA) for the patented minimally invasive SiLO […]
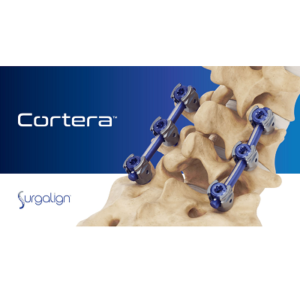
Surgalign Announces FDA 510(k) Clearance of the Cortera™ Spinal Fixation System – The Company’s New Flagship Posterior Fixation Platform
DEERFIELD, Ill., Aug. 24, 2022 (GLOBE NEWSWIRE) — Surgalign Holdings, Inc., (NASDAQ: SRGA) a global medical technology company focused on elevating the standard of care by driving the evolution of digital health, today announced FDA 510(k) clearance of the CorteraTM Spinal Fixation System. This new flagship product from Surgalign is a key piece to the foundational […]
Zavation Medical Products, LLC, receives FDA 510K Clearance for Varisync™ Anterior Cervical Plate and Spacer system as an addition to its cervical spine portfolio
FLOWOOD, Miss., Aug. 18, 2022 /PRNewswire/ — Zavation Medical Products (“Zavation” or the “Company”), an innovative designer and manufacturer of high-quality spinal implants, instruments, MIS procedural kits, and biologics headquartered in Flowood, MS, announced the FDA 510K Clearance of Varisync™, a cervical intervertebral body fusion device. Varisync™, the most recent addition to the Zavation cervical spine portfolio, has been tested and […]
Cutting Edge Spine Announces FDA 510(k) Clearance of T-FIX 3DSI Joint Fusion System with Proprietary Trabecular Technology
MINERAL SPRINGS, N.C. (PRWEB) JULY 14, 2022 Cutting Edge Spine (CES), a leader in the organic development and commercialization of bioactive implant technologies for the spine, today announced the FDA 510(k) clearance of its proprietary SI Joint Fusion System, T-FIX 3DSI Joint Fusion System. The 3D-printed T-FIX 3DSI Joint Fusion System is part of the novel EVOL-SI Fusion […]
SI-BONE, Inc. Announces FDA Clearance for Expanded Indication of the iFuse-TORQ® Implant System
SANTA CLARA, Calif., June 13, 2022 (GLOBE NEWSWIRE) — SI-BONE, Inc. (SIBN), (Nasdaq: SIBN), a Silicon Valley-based medical device company dedicated to solving musculoskeletal disorders of the sacropelvic anatomy, today announced an FDA clearance for iFuse-TORQ® for pelvic fracture fixation, including acute, non-acute and non-traumatic fractures.* Fractures covered in this clearance include pelvic fragility fractures (fractures related […]
Zavation Medical Products, LLC, receives FDA 510K Clearance for eZspand™ Lateral Cage as an addition to its eZspand™ Interbody System.
FLOWOOD, Miss., June 10, 2022 /PRNewswire/ — Zavation Medical Products (“Zavation” or the “Company”), an innovative designer and manufacturer of high-quality spinal implants, instruments, MIS procedural kits, and biologics headquartered in Flowood, MS, announced the FDA 510K Clearance of eZspand™ Lateral, an expandable lumbar interbody fusion device. The eZspand™ Lateral, part of the Zavation eZspand™ Interbody System, features unmatched expandable […]
Aurora Spine Corporation Receives FDA 510(k) Clearance for DEXA SOLO-LTM Anterior Lumbar Interbody Fusion Device as part of its DEXA Technology Platform
CARLSBAD, Calif, June 06, 2022 (GLOBE NEWSWIRE) — Aurora Spine Corporation (“Aurora Spine” or the “Company”) (TSXV: ASG) (OTCQB: ASAPF), a designer and manufacturer of innovative medical devices that improve spinal surgery outcomes, today announced that it had received FDA 510(k) clearance for its DEXA SOLO-L™ spinal fusion system. The 3D printed standalone anterior lumbar interbody […]
SMAIO Receives FDA 510(k) Clearance for Its Balance Analyzer 3D Surgery Planning Software
LYON, France–(BUSINESS WIRE)–Regulatory News: SMAIO (Software, Machines and Adaptative Implants in Orthopaedics – Euronext Growth Paris ISIN: FR0014005I80 / Ticker: ALSMA, eligible for PEA-PME equity savings plans), a French player specialized in complex spine surgery with a global offer comprising software, adaptative implants and related services, today announced that it has received FDA 510(k) clearance for its Balance Analyzer 3D […]
Stryker Gets FDA 510(k) Clearance for Q Guidance System
Stryker Corp. announced today that the Q Guidance System has received 510(k) clearance from the U.S. Food and Drug Administration. The company said its Q Guidance System, when used with the Spine Guidance Software, is an advanced planning and intraoperative guidance system designed to enable open or percutaneous computer-assisted surgery. Stryker said the Spine Guidance […]
SI-BONE, Inc. Receives FDA 510(k) Clearance for iFuse Bedrock Granite, a Breakthrough Pelvic Fixation and Fusion Technology
SANTA CLARA, Calif., May 31, 2022 (GLOBE NEWSWIRE) — SI-BONE, Inc., (Nasdaq: SIBN), a Silicon Valley-based medical device company dedicated to solving musculoskeletal disorders of the sacropelvic anatomy, announces FDA 510(k) premarket clearance of the iFuse Bedrock Granite Implant System (Granite). The Granite implant provides sacroiliac fusion and sacropelvic fixation as a foundational element for segmental […]
Accelus Receives U.S. FDA 510(k) Clearance for its Toro-L Interbody Fusion System
PALM BEACH GARDENS, Fla., May 19, 2022 (GLOBE NEWSWIRE) — Accelus, a privately held medical technology company focused on accelerating the adoption of minimally invasive surgery (MIS) as the standard of care in spine, today announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Toro Lateral (Toro-L) […]
FDA grants 510k clearance of the CTL Amedica Navigation Instrument System
DALLAS (PRWEB) MAY 18, 2022–CTL Amedica Corporation has received official 510k clearance from the U.S. Food and Drug Administration (FDA) to market the CTL Amedica Navigation Instrument System, registration number K213491. The system features manual surgical instruments adaptable for use with third-party navigation systems, which are designed to assist surgeons in precisely locating anatomical structures in […]