Caprera is a DLIF 3D Printed Titanium Cage with Expansion Feature. Caprera VIDEO ANIMATION Features: Excellent Stability: Additive manufacturing technology in combination with a unique geometrical implant design facilitates efficient and reliable primary and secondary fixation.Wide Variety of Footprints, Angles & Heights : The unique “net” structure, with its pore … [Read more...] about Caprera
Lateral and Anterior Expandable Cages
Giannutri
Giannutri es a DLIF 3D Printed Titanium Cage .Expandable.The design concept of Giannutri, 3D printed DLIF cage, is made to meet well experienced surgeons’ requirements. Tsunami Medical offers implants in a wide range of footprints, heights, and lordosis angles, providing just one system matching patients’ natural anatomy and surgeons’ preferences. For Giannutri, Tsunami … [Read more...] about Giannutri
AccelFix-XTP
AccelFix-XTP is an expandable cage specifically designed for the ATP approach.AccelFix-XTP Expandable Lateral Lumbar Cage System is composed of titanium alloy lateral cages which helps to restore disc height, segmental lordosis, and stability, while offering a customized fit for each patient’s unique anatomy. AccelFix-XTP VIDEO ANIMATION Features & … [Read more...] about AccelFix-XTP
AccelFix-XL
The AccelFix-XL expandable cage system for lateral approach helps restore disc height, segmental lordosis, and stability while offering a customized fit for the patient’s unique anatomy. AccelFix-XL VIDEO ANIMATION Features & Benefits Large graft window allows for greater graft volume to enhance fusion potentialSterile packaged implants help reduce surgical site … [Read more...] about AccelFix-XL
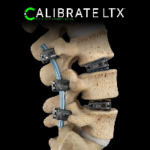
Calibrate LTXTM lateral expandable implant system
The Calibrate LTXTM is a lateral expandable implant system, technology designed to better achieve alignment goals with precise lordosis control and disc height restoration.The Calibrate LTX expandable lateral system seamlessly integrates with ATEC's entire lateral platform. The simplicity and ease of insertion of the implant is unique compared to the over-engineering that … [Read more...] about Calibrate LTXTM lateral expandable implant system
CALIBER®-L
CALIBER®-L is an expandable lateral lumbar fusion device designed to streamline insertion and optimize fit. Insertion of CALIBER®-L is performed at a contracted height to ease insertion. Controlled distraction is designed to maximize indirect decompression through disc height restoration. Continuous expansion resists migration by optimizing fit. CALIBER-L VIDEO ANIMATION … [Read more...] about CALIBER®-L
dualX™ Dual Expanding Interbody Fusion System
The dualX technology is comprised of a family of titanium expandable interbody devices designed to be implanted in lateral lumbar interbody fusion (LLIF), posterior lumbar interbody fusion (PLIF) and transforaminal lumbar interbody fusion (TLIF) spinal procedures. The dualX portfolio contains varying footprints, heights and degrees of lordosis with post-expansion bone grafting … [Read more...] about dualX™ Dual Expanding Interbody Fusion System
ELSA®
ELSA® is an expandable interbody fusion spacer with integrated fixation designed to maximize segmental lordosis while minimizing disruption to patient anatomy. ELSA VIDEO ANIMATION MARS™3V VIDEO ANIMATION MARS™ 3VL VIDEO ANIMATION Benefits: Adjustable Lordosis: Implants with adjustability from 5° to 20° or 15° to 30° allow for insertion at a smaller starting height … [Read more...] about ELSA®
ELSA®-ATP
ELSA®-ATP is an expandable lateral system designed to provide access to the lumbar spine anterior to the psoas muscle. Entering the disc space from this approach helps to avoid complications associated with the lateral trans-psoas approach and the lumbar plexus. This anterolateral working corridor also allows for direct access to L4‑L5 where a high iliac crest may complicate … [Read more...] about ELSA®-ATP
ORIGAMI Expandable Cage
Origami is a platform of Disruptive Designs for Expandable Interbody Cages. Origami VIDEO ANIMATIONOrigami-Lateral-Cages-Brochure.Twist Technologies.pdf Features: A Platform for open surgery or minimal accessfor TLIF, Lateral, ALIF & PLIF approachesdiverse choices of materials A Variety of Cage Designs 1 or 2 extensionsa choice of various … [Read more...] about ORIGAMI Expandable Cage
PROLIFT® Lateral
The PROLIFT e-LIF Spacer System with Osseo-Loc Surface Technology offers a Micro Invasive solution for Lateral Lumbar Interbody Fusions. With up to 6mm of in situ expansion and up to 15˚ of lordosis, ProLift allows for restoration of patient disc height and decompression of neural elements. With the small starting height options and maximum end plate coverage, it is … [Read more...] about PROLIFT® Lateral
ProLift Lateral HELO
ProLift Lateral HELO with Osseo-Loc Surface Technology offers a micro invasive solution for Lateral Lumbar Interbody fusion. With in situ lordotic expansion from 5° up to 20°, ProLift Lateral HELO allows for sagittal balance and lordosis correction. With the small starting height options and maximum end plate coverage, it is designed to minimize … [Read more...] about ProLift Lateral HELO
RISE® L
RISE® L is an expandable lateral lumbar fusion device that offers up to 7mm of expansion coupled with a large graft chamber and the ability to introduce autogenous bone graft in situ. RISE®-L VIDEO ANIMATION MARS™3V VIDEO ANIMATION MARS™ 3VL VIDEO ANIMATION The titanium implant provides continuous in situ expansion and is designed to restore disc height with proper … [Read more...] about RISE® L
Sagittae® Expandable Lateral Lumbar Fusion Device
Sagittae® is a lateral lumbar interbody with independent height and lordosis adjustment designed to restore sagittal balance. Sagittae VIDEO ANIMATIONSagittae.Brochure-(SpinEx)Adcura Spine.pdf Features: Patient-specific In-situ parallel expansion up to 16.1mm Independent lordotic adjustment up to 30 degrees* Designed for Surgical Efficiency Single … [Read more...] about Sagittae® Expandable Lateral Lumbar Fusion Device
SAHARA® Lateral 3D Expandable Interbody System
SAHARA Lateral is the first ever 3D-printed lateral expandable fusion device and features passive expansion capabilities that are designed to allow surgeons to achieve up to 30 degrees of sagittal spinal correction in skeletally mature patients.Utilizing a passive expansion mechanism, the implant can either be adjusted from a lateral approach intraoperatively or can adjust … [Read more...] about SAHARA® Lateral 3D Expandable Interbody System
StaXx® IBL Expandable Interbody Device
The StaXx® IBL Expandable Interbody Device’s low profile, expandable design, is designed to mitigate subsidence and maximize indirect decompression. The StaXx® IBL Expandable Interbody Device is intended to be introduced to the disc space via the lateral approach. StaXx IBL VIDEO ANIMATION Benefits: Mitigate subsidence (reduced endplate stress/micro fracturing); maximize … [Read more...] about StaXx® IBL Expandable Interbody Device
LONGBOW™
The innovative LONGBOW Expandable Lateral Spacer System is the first interbody on the market that expands laterally (A/P) in-situ specifically for a direct lateral approach. The benefits of LONGBOW are transferred directly to the patient by minimizing tissue retraction and potential nerve damage associated with the lateral access approach. LONGBOW VIDEO … [Read more...] about LONGBOW™
Torus-SA
Torus-SA is a 3D Printed Expandable Stand-Alone lumbar cage. This product is not cleared by the FDA for distribution in the U.S. Features: Titanium Alloy Implant with 3D Printed Textured Titanium Endplates Promotes Immediate Mechanical Fixation & Potentially Upregulating the Production of Osteogenic Factors that are Critical for Bone Growth & Fusion … [Read more...] about Torus-SA
Torus
Torus is a 3D Printed Expandable lumbar cage. This product is not cleared by the FDA for distribution in the U.S. System Features About NeuroStructures NeuroStructures is a medical device company focused on designing, developing, manufacturing and marketing proprietary, high-quality medical device systems. All of our products provide comprehensive medical solutions … [Read more...] about Torus