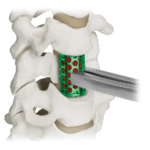
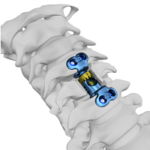
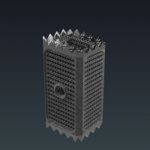
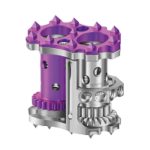
COLOSSEUM™ is a titanium mesh vertebral body replacement spacer engineered to reduce procedural steps and streamline the static corpectomy procedure. Straight or curved spacers and a variety of endcap angulations are designed to accommodate varying patient anatomy, optimize fit. COLOSSEUM VIDEO ANIMATION Features and Benefits: Incrementally Pre-Cut Spacers: Allow … [Read more...] about COLOSSEUM™
CORPECTOMY DEVICES
DISRACTANIA SPINAL Cage
About ARTFX Medical ARTFX Medical is a privately held company located in the U.S.A. We manufacture the highest quality, safe, effective, and affordable spinal and trauma implants, as well as surgical instruments. We offer an extensive array of innovative, surgeon focused systems designed with the best clinical outcomes in mind. Our various devices are CE or FDA approved … [Read more...] about DISRACTANIA SPINAL Cage
DAS V2 ULTRA
DAS V2 ULTRA is a cervical expandable corpectomy cage integrated with ananatomically contoured plate with Quick login Feature and an ultra-fast controlled implant expander instrument for quick implantation. Built-in plate eliminates the requirement for additional platingImplant Insertion and ultra-fast expansion with the same instrument reduces the operative timeQuick … [Read more...] about DAS V2 ULTRA
DAS ULTRA
DAS ULTRA is a cervical expandable corpectomy cage integrated with ananatomically contoured plate with a one-step locking feature and an ultra-fast controlled implant expander instrument for quick implantation. Built-in plate eliminates the requirement for additional platingImplant Insertion and ultra-fast expansion with the same instrument reduces the operative timeOne-step … [Read more...] about DAS ULTRA
ECD – Expandable Corpectomy Device
ECD is a vertebral body replacement for the cervical and upper thoracic spine. Its expansion mechanism is designed to allow smooth, continuous expansion in situ.Radiolucent PEEK implants with different heights and endplate angulations are designed to enable the surgeon to choose the specific configuration suited to the individual pathology and anatomical condition. ECD – … [Read more...] about ECD – Expandable Corpectomy Device
ESPINAX SPINAL Cervical Corpectomy Cage
About ARTFX Medical ARTFX Medical is a privately held company located in the U.S.A. We manufacture the highest quality, safe, effective, and affordable spinal and trauma implants, as well as surgical instruments. We offer an extensive array of innovative, surgeon focused systems designed with the best clinical outcomes in mind. Our various devices are CE or FDA approved … [Read more...] about ESPINAX SPINAL Cervical Corpectomy Cage
FORTIFY®
The FORTIFY® adjustable corpectomy spacer streamlines vertebral body replacement through the desired approach and provides a multitude of options designed to restore height, alignment and stability. FORTIFY VIDEO ANIMATION FORTIFY® is available in a wide variety of footprints, heights, lordotic/kyphotic angles, and can accommodate various approaches. In addition, … [Read more...] about FORTIFY®
FORTIFY® I
FORTIFY® I is an integrated vertebral body replacement device that provides anterior column support and is designed to prevent implant dislodgement. The spacer has integrated titanium plates and screws for additional stabilization between the vertebral bodies and the spacer. The implants are available in a wide range of footprints, heights, and lordotic/kyphotic angles in PEEK … [Read more...] about FORTIFY® I
FORTIFY VA™
FORTIFY® Variable Angle is a self-aligning expandable corpectomy spacer that optimizes anatomical fit through an anterior cervical approach or one of several thoracolumbar approaches. Features: Self-Aligning Polyaxial Endplate: Designed to optimize load distribution between the implant and vertebrae.Expandable Core: Allows for continuous expansion to the desired … [Read more...] about FORTIFY VA™
GIZA™
The GIZA™ is the latest generation of titanium expandable Vertebral Body Replacement Systems.It allows surgeons to perform lumbar or thoracic Corpectomy procedures, and has been specifically developed to improve patient care by saving valuable time OR time and decreases the risk of subsidence. GIZA VIDEO ANIMATION (Eden Spine) Giza.Brochure-Stryker.pdf … [Read more...] about GIZA™
Hawkeye TI
Hawkeye TI is changing the game for cervical corpectomy cases. With its 510(k) cervical clearing, 8° lordosis, and multiple footprints corpectomy cases are now more efficient than ever. Hawkeye TI is getting surgeons and patients out of the operating room and on the road to recovery faster and more efficiently. Hawkeye VIDEO … [Read more...] about Hawkeye TI
Hydrolift®
Hydrolift® is the first vertebral body replacement system that is distracted not mechanically but hydraulically with sterile saline water. The slim implant body allows a bone saving partial corpectomy. The anatomical endplates with the proven Plasmapore® coating guarantee a save and quick osteointegration. The endplates can adapt freely in a ±10° angulation and can be adapted … [Read more...] about Hydrolift®
KONG®-TL VBR
The rough Ti-iT® titanium coating and the radiolucent BlackArmor® material make the KONG®-TL VBR E vertebral body replacement a unique implant for the thoracolumbar spine. In particular, the KONG®-TL VBR E paves the way for new perspectives and treatment approaches in tumor/radiation therapy. KONG-TL VBR.Brochure.Icotec.pdf Benefits: Radiolucent BlackArmor® material … [Read more...] about KONG®-TL VBR
KONG®-C VBR M
The rough Ti-iT® titanium coating and the radiolucent BlackArmor® material make the KONG®-C VBR M vertebral body replacement a unique implant for the cervical spine. In particular, the KONG®-C VBR M paves the way for new perspectives and treatment approaches in tumor/radiation therapy. KONG-C_VBR-Brochure.Icotec.pdf Benefits: Radiolucent BlackArmor® material … [Read more...] about KONG®-C VBR M
LorX® Corpectomy Cage System
The LorX® Corpectomy Cage System (LorX Expandable Corpectomy Device and LorX Titanium Corpectomy Mesh Device) are vertebral replacement implants for the cervical, thoracic and lumbar spine (C3-L5) to replace adjacent vertebrae (max. 3 levels). LorX-Corpectomy-Brochure.Tria-Spine.pdf Features: Titanium MaterialAnatomical designDistractableDifferent Dimensions … [Read more...] about LorX® Corpectomy Cage System
LEANDER® vertebral body replacement
The LEANDER® vertebral body replacement is an implant system that is suitable for long-term use in anterior stabilisation of the cervical spine; it can replace one or more vertebral bodies in patients whose general skeletal growth has ceased. The system is intended for surgical treatment of tumorous, inflammatory and traumatic diseases as well as injuries of the cervical spine … [Read more...] about LEANDER® vertebral body replacement
Matrixx Corpectomy
NEXXT MATRIXX® is a collection of additively manufactured spacers for cervical, lumbar/lumbosacral and thoracolumbar implantation. The basic shape of these implants is a structural column to provide surgicalstabilization of the spine. Each device comprises an external structural frame having a roughened surface (~7μm). The intervening geometric lattices have pores 300-700μm. … [Read more...] about Matrixx Corpectomy
MaxFuse® PEEK VBR System
The MaxFuse® PEEK VBR system is used to replace a diseased vertebral body, resected or excised for the treatment of tumors, to achieve anterior decompression of the spinal cord and neural tissues in the thoracolumbar spine. The device also aids in restoring the height of a collapsed vertebral body. The system is made from PEEK-OPTIMA® from Invibio® Biomaterial Solutions, a … [Read more...] about MaxFuse® PEEK VBR System
mediExpand®-TL
The expandable vertebral body replacement "mediExpand®"-TL was developed for reconstruction of the thoracic and lumbar spine after single or multilevel corpectomies as a result of tumors. Development partner PD Dr. med. Heinrich Böhm Benefits: Two opposed threaded columns allow a variable extension and can be adjusted individually Depending on the selected … [Read more...] about mediExpand®-TL