ANTERIOR INTERSOMATIC CAGE Features: SAGA ANTERIOR LORDOTIC INTERSOMATIC CAGE About Vincula We are a Brazilian multinational company committed to promoting the well-being of the individual through solutions in accessible medical devices and with an international quality standard.The Vision is to be the best option in orthopedics, spine, trauma and … [Read more...] about Fusimax/Saga
Anterior Lumbar Interbody Fusion Cages (ALIF)
Leva® AF Anterior Stand-Alone Spacer System
The Leva® AF Interbody Device features all-titanium construction, modular anterior plates and a selection of bone screws for integrated and stand alone vertebral body fixation. As with all Leva® brand products, the device also offers a large graft chamber that facilitates copious grafting with complete graft-to-endplate contact within the device. The Leva® AF … [Read more...] about Leva® AF Anterior Stand-Alone Spacer System
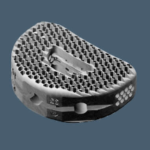
HEDRON IA™ 3D Printed Integrated ALIF Spacer
HEDRON IA™ is a 3D Printed Integrated ALIF spacer that features a biomimetic porous scaffolding designed to encourage cellular response and promote bone formation onto and through the implant. HEDRON VIDEO ANIMATION HEDRON™ spacers feature a biomimetic porous scaffolding designed for bone formation onto and through the implant. HEDRON P™ implants are available in a wide … [Read more...] about HEDRON IA™ 3D Printed Integrated ALIF Spacer
kili Anterior lumbar interbody fusion cage
kili implants are an internal spinal fixation system intended to provide stabilization of the lumbar spine while biologic fusion occurs.The kili cages are inserted by impaction in the discal space by anterior approach. The kili screws can be added for better anchoring.kili implants are used with dedicated instruments which allow preparation of the site, insertion and removal of … [Read more...] about kili Anterior lumbar interbody fusion cage
Idys®-ALIF ZP 3DTi
The design allows four fixation screws to be inserted through the cage and into the adjacent vertebral bodies to create a zero-profile stand-alone construct that removes the need for posterior fixation. Idys®-ALIF ZP 3DTi is available in sterile packaging and available in multiple footprint, heights and lordotic angles. Idys®-ALIF ZP 3DTi VIDEO ANIMATION Benefits: Zero … [Read more...] about Idys®-ALIF ZP 3DTi
IdentiTi™-ALIF LW
ATEC’s IdentiTi Anterior Lumbar LW Porous Ti Interbody Implants are designed to provide the biological, biomechanical, and imaging characteristics that surgeons seek in a fusion construct. The subtractive process used to manufacture each IdentiTi Implant results in more predictable mechanical performance and enhanced imaging characteristics. IdentiTi implants take advantage of … [Read more...] about IdentiTi™-ALIF LW
JULIET®AN
The JULIET®AN is a lumbar anterior cage from SpineArt. Benefits: Anatomical shape: The implant’s sloping anatomical shape is designed to facilitate the insertion and to allow 2 approaches: anterior and antero-lateral. Load sharing concept: JULIET®AN’s slits are designed to enhance flexion of the device under a physiological compressive load. This concept is intended to … [Read more...] about JULIET®AN
MectaLIF Anterior Interbody Fusion Device
The Medacta MectaLIF Anterior Interbody Fusion Device offers an unprecedented MODULAR design that incorporates the benefits of an anterior plate and a radiolucent interbody spacer.In order to accommodate specific anatomical requirements and specific pathologies to treat, the surgeon has the ability to assemble any of the available plates intraoperatively giving the complete … [Read more...] about MectaLIF Anterior Interbody Fusion Device
MAGNIFY®-S
MAGNIFY®-S is an all titanium expandable ALIF device designed to maximize indirect decompression, restore disc height, and optimize fusion potential. Minimized insertion height and continuous expansion combined with the ability to pack additional bone graft after expansion makes MAGNIFY®-S an ideal choice for stand-alone ALIFs. Benefits: Maximized Indirect … [Read more...] about MAGNIFY®-S
MAGNIFY®
The MAGNIFY® expandable ALIF spacer system is designed to minimize impaction, maximize indirect decompression, and optimize fusion. Minimized insertion height, continuous expansion, and the ability to backfill autogenous bone graft separates MAGNIFY® from from traditional ALIF spacers. Benefits: Minimize Impaction: The reduced insertion height eases … [Read more...] about MAGNIFY®
MONDRIAN™ ALIF Cage System
The MONDRIAN™ ALIF Cage System with Supplementary Fixation Plate, designated K213641/S003, is an integrated plate-and-cage construct, that can be manufactured in PEEK, titanium and a hybrid titanium-plated PEEK. The system, a standalone extension to the already robust MONDRIAN™ offering, features CTL Amedica’s proprietary TiCro™ surface technology, which significantly increases … [Read more...] about MONDRIAN™ ALIF Cage System
Hygro®-L ALIF
Hygro-Cages consist of an additive manufactured honeycomb titanium structure with vertically and horizontally connected pores.This structure showed prove of it´s suitability for spinal fusion in a clinical PMCF-study1 and therefore it can be concluded that cell transfusion into the cage structure is supported. The capillary effect due to the specifically designed pore … [Read more...] about Hygro®-L ALIF
MONUMENT
MONUMENT is a unique ALIF system with an integrated mechanical reduction feature that is designed to aid in spondylolithesis reduction (Grade 1). MONUMENT VIDEO ANIMATION Benefits: 8mm of Translation: Integrated mechanical reduction feature allows up to 8mm of continuous translation Enhanced Endplate Fixation: Implant adjustability allows for optimal cortical bone … [Read more...] about MONUMENT
Meridian with WaveForm A
The Meridian® ALIF system was designed to be a modular instrument and implant system to streamline the ALIF procedure and provide diverse fixation options for single to multilevel ALIFs in a reduced number of sets. Implants feature WaveForm® technology, the next level of 3D printed innovation designed to prioritize strength, surface, and stability. With a repeating and … [Read more...] about Meridian with WaveForm A
Modulus ALIF
Benefits: About NuVasive NuVasive, Inc. (NASDAQ: NUVA) is transforming spine surgery and beyond with minimally invasive, procedurally-integrated solutions designed to deliver reproducible and clinically-proven surgical outcomes. The Company’s portfolio includes access instruments, implantable hardware, biologics, software systems for surgical planning, navigation … [Read more...] about Modulus ALIF
Monterey AL Interbody System
Monterey AL Interbody System, is a stand-alone interbody fusion device designed for anterior lumbar interbody fusion (ALIF). Monterey AL is made up of both solid and porous structures within a single implant, leveraging Stryker’s proprietary Tritanium In-Growth Technology, a material designed to mimic cancellous bone and provide an environment favorable to bone regeneration and … [Read more...] about Monterey AL Interbody System
MANTIZ Medical: PANTHER 3D Printed ALIF Cage
The PANTHER 3D printed range of cages have been designed to minimize the subsidence by adopting the cross intersected ring frame. PANTHER VIDEO ANIMATION Features: Reduction of dislocation due to rough surface of the cages and top bottom teeth.Minimized subsidence due to elasticity of RING FRAME MESH structureIncreased fusion effect as it is very similar to the … [Read more...] about MANTIZ Medical: PANTHER 3D Printed ALIF Cage
MAGNUM+
Magnum+ is the Stand-Alone, No Profile ALIF from Spinal Elements.It is an Advanced Locking ALIF System with Titanium Porous Coating. MAGNUM VIDEO ANIMATION MAGNUM.Brochure-Spinal elements.pdf Benefits: Minimize Complexity + Locking Screws + Lag Compression + Multiple Insertion Options + Controlled Delivery + Mechanical Stability Maximize Success + Immediate Stability + … [Read more...] about MAGNUM+
M3 Stand-Alone ALIF System
The M3 Stand-Alone ALIF System features a versatile, diverging 3-screw construct that incorporates the biocompatible benefits of Mimetic Metal™ and StrutSure™ technologies, along with simplified instrumentation. Features: 100% open-pore architecture to allow for osteointegrationHydrophilic wicking3D printed titanium with 70% porosityStrutsure:Dual-zone microdeflection … [Read more...] about M3 Stand-Alone ALIF System