MEMPHIS, Tenn.–(BUSINESS WIRE)–Dr. Raymond Gardocki of the Campbell Clinic in Memphis, TN, performed his 100thendoscopic spine surgery on February 6, 2019, utilizing joimax® endoscopic spine technologies. By implementing joimax®endoscopic surgical systems into their center, Dr. Gardocki and the Campbell Clinic are on the forefront of minimally invasive spine surgery. “We are very excited to have completed […]
2019
Xtant Medical Announces Appointment of New Senior Sales Leaders
BELGRADE, MT, Feb. 12, 2019 (GLOBE NEWSWIRE) — Xtant Medical Holdings, Inc. (NYSE American: XTNT), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, announced today the recent additions of five senior sales executives: Dana Lyons, Vice President of US Sales, Jamey Rottman, Regional Vice President, Mid Atlantic, Chris […]
Orthobion awarded as Best Spinal Treatment Product Development & Marketing Specialists 2018
Founded in 2009, Orthobion is a privately funded start- up specialising spinal treatment options, especially spinal fusion devices.Today, Orthobion offers a complete line of interbody fusion devices featuring its unique FGOIC Ti surface technology, which are designed to promote bone growth, optimized intervertebral stability, while allowing for improved bone healing characteristics. Among these solutions is […]
Smith & Nephew Drops on Report of Deal Talks With NuVasive
(www.bloomberg.com)–Smith & Nephew Plc dropped on a report that the medical-device manufacturer is in talks with NuVasive Inc. about a deal worth more than $3 billion to gain spinal surgery products. The stock fell as much as 5.5 percent in London Monday. NuVasive share rose 11 percent to $54.85 at 9:57 a.m. in New York, after a report Friday in […]
Alphatec Announces Stay of Court Proceedings in NuVasive Patent Lawsuit
CARLSBAD, Calif., Feb. 11, 2019 (GLOBE NEWSWIRE) — Alphatec Holdings, Inc. (“ATEC” or “the Company”) (Nasdaq: ATEC) today announced several favorable developments in its ongoing patent litigation with NuVasive, Inc. (Nasdaq: NUVA). On February 6, 2019, the United States District Court for the Southern District of California (the “Court”) granted a stay in the patent […]
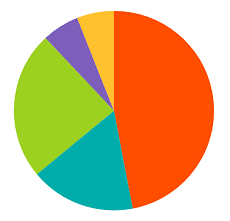
Which is the Spine Market Estimate for 2019?
The Spine market accounts in 2019 for more than $9 billion worldwide, with an estimated increase of 3% vs. 2018. Our estimate of the 10 top spine players’ market share in 2019 is the following: Medtronic:With the acquisition of the Mazor robot, Medtronic Spine is trying to increase sales (our estimate for 2019 is a […]
Smith & Nephew In Talks To Buy NuVasive For Over $3 Bln
(RTTNews) – Smith & Nephew (SN.L) has held talks to buy medical instruments maker NuVasive in a deal that would be worth more than $3 billion, the Financial Times reported,citing people with direct knowledge of the talks. The exact terms of any discussions could not be learned and talks between the two sides may fall […]
joimax® Announces New Distribution Partners in Brazil and Japan
KARLSRUHE, Germany–(BUSINESS WIRE)–joimax®, the Germany-based market leader of technologies and training methods for full-endoscopic minimally-invasive spinal surgery, is pleased to announce it has expanded its international market presence with new distribution partners in Brazil and Japan. In Brazil, joimax® is partnering with the privately-owned CT Group, which offers more than 20 years experience in the medical technology […]
ISASS Recommendations and Coverage Criteria for Bone Graft Substitutes used in Spinal Surgery
Autologous bone graft remains the gold standard by which bone graft substitutes are compared in spine fusion surgery. The utilization of bone graft substitutes, either as (1) an extender for spinal fusion constructs or (2) an alternative to minimize morbidity while maximizing outcomes, is changing. Moreover, current procedures technology (CPT) code 20939 became effective in […]
Dr. Robert Watkins, Jr. has officially joined the Osseus Fusion Systems Aries-L alpha launch team!
DALLAS, TX – February, 7, 2019 – Dr. Robert Watkins, Jr. has officially joined the Osseus Fusion Systems Aries-L alpha launch team! He performed his first surgery with the titanium 3D printed lateral interbody device on January 24, 2019. Dr. Watkins has been a fundamental partner – assisting Osseus Fusion Systems in the conceptualization and […]
Orthofix Announces FDA Approval of the M6-C Artificial Cervical Disc to Treat Patients with Cervical Disc Degeneration
LEWISVILLE, Texas–(BUSINESS WIRE)–Orthofix Medical Inc. (NASDAQ:OFIX), a global medical device company focused on musculoskeletal products and therapies, today announced U.S. Food and Drug Administration (FDA) approval of the M6-C™artificial cervical disc for patients suffering from cervical disc degeneration. The M6-C artificial cervical disc was developed by Spinal Kinetics, a company acquired by Orthofix in April […]
KICVentures Announces Three New Developments in Spine Surgery
MALDEN, MASS. (PRWEB) FEBRUARY 05, 2019–KICVentures, a Boston-based private investment firm leading in healthcare technology, announced today three exciting new developments in spine surgery in the areas of Motion Preservation and Biologics. The first development is AxioMed’s viscoelastic total spinal disc replacement. It was first developed by Dr. Art Steffee who conceived the idea of […]
Nanovis Wins the Global Health & Pharma 2018 Technology Awards
CARMEL, IND. (PRWEB) FEBRUARY 05, 2019–Nanovis today announced that Global Health & Pharma magazine recognized Nanovis as the Best Nanotechnology Driven Implant Company, 2018. Nanovis is a technology-driven growth company committed to helping surgeons and hospitals achieve excellent fixation and infection outcomes using advanced nanotechnology platforms. Its industry-leading fixation technologies offer surgeons and hospitals the […]
Orthofix Acquires Options Medical
LEWISVILLE, Texas–(BUSINESS WIRE)–Orthofix Medical Inc. (NASDAQ:OFIX), a global medical device company focused on musculoskeletal products and therapies, today announced that it has acquired the business of Options Medical, LLC, a medical device distributor based in Florida. “Options Medical has been a successful distributor for our Bone Growth Therapies devices for many years,” said Brad Niemann, […]
Additive Manufacturing is the TREND!Which are the 27 Cages that will compete in the market in 2019?
Additive Manufacturing (Three-dimensional printing 3DP), is a very rapidly growing industry trend in the area of spinal surgery. The introduction of additive manufacturing into the spinal industry started a revolution enabling increased complexity of implant design and patient-specific solutions. Why Additive Manufacturing is the TREND and the Leading Companies are launching 3D Cages? Porous titanium […]
Camber Spine Reports Significant Fourth Quarter And Full Year Sales Results
KING OF PRUSSIA, Pa., Jan. 31, 2019 /PRNewswire/ — Camber Spine, a leading innovator in spine and medical technologies, today reported preliminary unaudited proprietary sales results (sales of products innovated and owned by the company) for the fourth quarter and full year ending December 31, 2018. These preliminary unaudited results include: Fourth quarter 2018, proprietary […]
Genesys Spine wins an iF DESIGN AWARD 2019
AUSTIN, Texas, Jan. 31, 2019 /PRNewswire/ — Genesys Spine’s AIS-C Stand-Alone System was a winner of this year’s iF DESIGN AWARD, a world-renowned design prize. Each year, the world’s oldest independent design organization, Hannover-based iF International Forum Design GmbH, organizes the iF DESIGN AWARD. The AIS-C Stand-Alone System won over the 67-member jury, made up […]
iNetworks invests in Wenzel Spine
Pittsburgh-based iNetworks LLC on Wednesday said it has invested $1 million into an Austin, Texas-based medical technology company, leading its financing round. Wenzel Spine Inc. provides minimally invasive surgical solutions for the treatment of spinal disorders, simplifying procedures and reducing recovery time. “We are excited to be an investor and a strong partner to the […]
Southern Spine Announces New Thoracic Dual Lamina Implants for StabiLink® Interlaminar System and Additional US Patents
MACON, Ga.–(BUSINESS WIRE)–Southern Spine, LLC, an ISO 13485:2016 certified manufacturer of implants and instruments for spinal surgery, announced today the release of three (3) new StabiLink® Dual Lamina Implants specifically designed for upper thoracic clinical use. Alan H. Daniels MD, Associate Professor of Orthopaedic Surgery at The Warren Alpert Medical School of Brown University has specifically used the StabiLink® Dual […]
Implanet Confirms the Reorganization of Its Sales and Reports Full-Year 2018 Revenue
Implanet (Paris:ALIMP) (OTCQX:IMPZY) (Euronext Growth: ALIMP, FR0010458729, eligible for PEA-PME equity savings plans), a medical technology company specializing in vertebral and knee surgery implants, confirms the implementation of a reorganization of its sales force with the introduction of direct sales, and reports revenue for the fourth quarter and the year ended December 31, 2018. Sales […]
Medtronic Announces U.S. Commercial Launch of Mazor X Stealth(TM) Edition for Robotic-Assisted Spine Surgery
DUBLIN – January 28, 2019 – Medtronic plc (NYSE:MDT) today announced the first U.S. patients treated with the Mazor X Stealth(TM) Edition for spine surgery following its recent commercial launch. The Mazor X Stealth Edition offers a fully-integrated procedural solution for surgical planning, workflow, execution and confirmation. The system was first used at Norton Healthcare in […]
New Study Finds Plasmapore®XP Surface Enhancement Demonstrates Enhanced Osseointegrative Properties
CENTER VALLEY, Pa., Jan. 28, 2019 /PRNewswire/ — Aesculap Implant Systems, LLC today announced the publication of “Porous titanium-coated polyetheretherketone implants exhibit an improved bone–implant interface: an in vitro and in vivo biochemical, biomechanical, and histological study” in Medical Devices: Evidence and Research.1 The paper combined in vitro and in vivo analysis to examine the PlasmaporeXP technology (Aesculap AG, Tuttlingen, Germany), a porous titanium surface enhancement that […]
25 Innovative Corpectomy Devices. The Market? More than 150,000 spinal fractures in North America every year
Each year, there are more than 150,000 spinal fractures in North America* that sometimes may require additional spinal structural support with a Mesh or with an expandable corpectomy device. Another indication or use for these Devices is in the treatment of cancer patients. If the cancer metastasizes or spreads in the spine, the surgeon may […]
We are proud and happy to announce that SpineGuard is again Sponsor of SPINEMarketGroup in 2019!
Thank you SpineGuard! On behalf of TheSPINEMarketGroup team, we would like to thank you for your GOLD Sponsorship and for your support this year 2019 again. About SpineGuard Pierre Jérôme and Stéphane Bette founded SpineGuard in January 2009 with the goal of delivering the clinical benefits of the DSG™ (Dynamic Surgical Guidance) technology to as […]
Globus Medical’s SECURE®C Cervical Artificial Disc Receives Expanded Insurance Coverage
AUDUBON, Pa., Jan. 24, 2019 (GLOBE NEWSWIRE) — Globus Medical, Inc. (NYSE:GMED), a leading musculoskeletal solutions company, recently announced that the SECURE-C® Cervical Artificial Disc is now covered by Anthem, one of the largest health benefits companies in the United States with close to 40 million medical members and over 73 million lives covered. Approved by […]
Bone & Joint Institute of South Georgia Performs First Endoscopic Spine Surgery in the U.S. with the New joimax® 4K Endoscopic Spine Surgery System
JESUP, Ga.–(BUSINESS WIRE)–Dr. Thomas Loumeau of the Bone & Joint Institute of South Georgia, performed the first endoscopic spine surgery on January 8, 2019 using the newly launched 4K Endoscopic Spine Surgery System from joimax, Inc. The new 4K endoscopic system gives the Bone & Joint Institute the advantage of being on the cutting edge of […]
SpineGuard Reports 2018 Revenue of €7.6M
SpineGuard (Paris:ALSGD) (FR0011464452 – ALSGD), an innovative company that designs, develops, and markets disposable medical devices intended to make spine surgery safer by bringing real-time digital technology into the operating room reported today its preliminary unaudited consolidated full-year 2018 revenue, EBITDA and cash position. Stéphane Bette, CEO and co-founder of SpineGuard, said: “In 2018, SpineGuard successfully […]