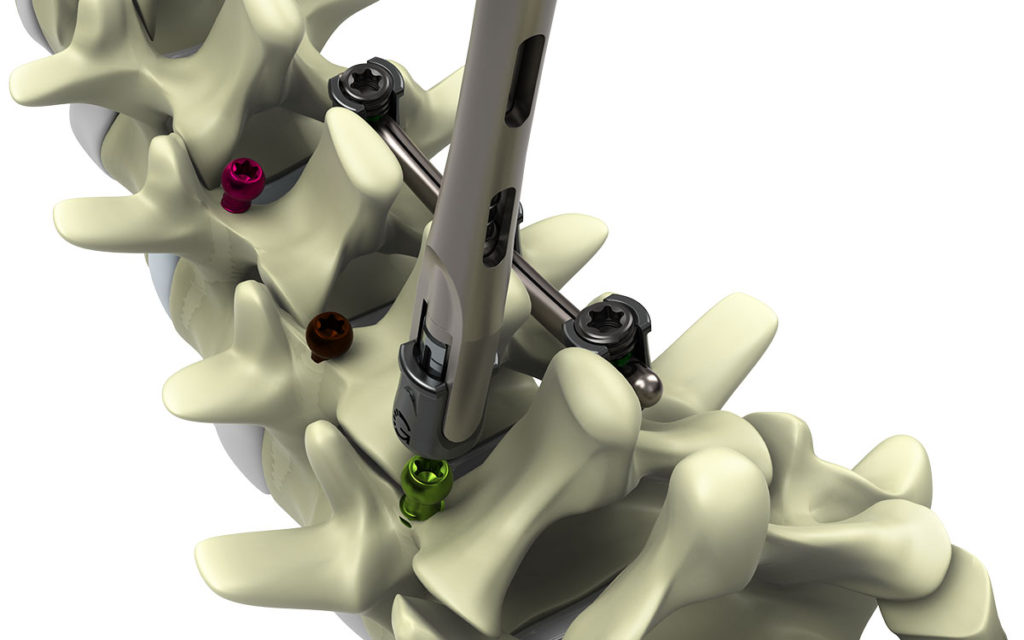
The Genesys Spine TiLock® Modular Spinal System uniquely addresses the challenges of posterior fixation by providing surgeons with the flexibility to secure difficult operative levels.
Benefits:
- The TiLock® Modular Spinal System can be implanted via a conventional open approach, midline, or minimally invasive.
- Increased options for distraction, greater visibility, and more thorough decortication may be achieved by allowing the surgeon to first place a low profile screw.
- The reduced tulip head profile allows for efficient screw placement with minimal bony removal helping to mitigate the risk of adjacent facet encroachment.
- The system is designed to minimize the retraction and disruption of surrounding tissue, and maximize fixation in denser cortical bone.
- Proprietary Opposing Angle Interface (OAI) Set Cap. The lock system reduces rod slippage as well as incidences of set cap back out.
About Genesys Spine
Founded in December of 2009, Genesys Spine’s mission was to bring a suite of medical implants and instruments with novel characteristics into a mature spinal fusion market. Since that time, Genesys has released four new FDA cleared product lines with several other products in various stages of the design and development cycle. Genesys has also developed several proprietary instruments to aid in the use of its products. All of Genesys’ inaugural products including our system specific instruments, have garnered very positive feedback from the marketplace. Our implants, with their proprietary features, all remain within existing parameters for today’s current reimbursement codes.
For more information please visit: http://www.genesysspine.com
