10 years ago, in 2010 a French company launched the first single-use spine system creating a lot of expectation and also a lot of skepticism about the future of this new concept. Since then, Safe Orthopedics has not stopped growing and several competitors have also appeared on the market. Thus, in 2011 Xenco Medical was founded, also focusing on single-use spine systems that even recently have dared to launch the first interactive vending machines designed for spine surgery instruments and implants.
Since then, several companies specializing in disposable spine systems have appeared, such as Neo Medical recently, which already in the fall of 2017, received FDA approval for the US market. The first operation performed with Neo components in the US was in the first quarter of 2018. A couple of months ago, last September, Orthofix and Neo Medical Announced Partnership to Develop and Market Single-Use Spinal Solutions Focused on Improving Patient Outcomes.
Today there are at least 8 companies that offer single use spinal instruments which are the following: Single-use Companies
- Xenco Medical
- Safe Orthopaedics
- Providence Medical Technology: CORUS Spine System
- Neo Medical
- Intelligent Implant Systems
- Evospine
- ECA Medical Instruments
- STERILE SPINE™ Anterior Cervical Plate (ACP) System
But does it really bring advantages? What are the PROS and CONS of these systems?
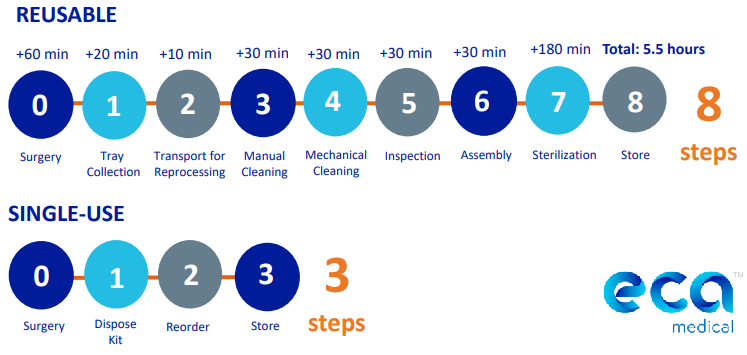
- More Productivity: According to a ECA Medical, in a single-blind interview of ASC owners all respondents rated “time efficiencies to perform more cases” as the most important reason to using single-use procedure kits. Reprocessing adds 5.5 hours to instrumentation preparation time for surgery and adds human error with the 8 steps required. These times are exacerbated when instrumentation issues occur (dirty, missing or damaged). For simple, high volume procedures, this inefficiency can be eliminated.
- Less Risk of Infections: Reduced risk of surgical site infections
- Less Inventory: Thoracolumbar fixation systems currently have excessive inventory requirements: 3-5 instruments trays or more and 400+ types of screws.
Undoubtedly, the advantages are many but they are focused mainly on the manufacturer, the hospital and the nursing staff.
But, are these systems really advantageous for the surgeon? In short degenerative surgeries, its usefulness can be great since it provides speed and efficiency. But in more complex surgeries, simplicity becomes a drawback with fewer instruments and possibly inferior quality. On the other hand, the number of implants is also limited, thus providing fewer resources for surgery.
Without a doubt, single-use systems are here to stay and today they already have their place in the market. We are sure that in the future, most companies will offer this type of product at least for simple or easy to standardize surgeries.
