In recent years, we have all witnessed the maturity or stabilization of the market for interspinous implants. However, TMR released a study in which it estimates that it will continue to grow significantly during the next few years.
Why is this market growing?
The global interspinous spacers market will continue to grow because of the rise in the elderly population and the launch of new innovative products.
- Increasing Elderly Population
- Spine disorders such as degenerative disc diseases and spinal stenosis are frequent among aged people. Their growth in the US will increase the demand for spine surgeries. For instance, according to the Centers for Disease Control and Prevention (CDC), the number of elderly populations aged 65 years or above will double to about 71 million by 2030. In these patients, lumbar spinal stenosis (LSS) is considered the most frequent cause of spinal surgery. For this indication, interspinous implants are one of the main solutions to relieve discomfort related to LSS.
- Launch of New and Innovative Products
- Technological advances and the introduction of new products all over the world are also important factors supporting the interspinous spacer market growth. Many key market leaders invest in R&D to create and introduce new and innovative devices. Additionally, 3D printing technology facilitates rapid prototyping of surgically implantable products, such as interspinous spacers. Due to the increasing demand for personalized care, the manufacturers of orthopedic devices are competing to provide patient-specific 3D-printed spinal implants.
Which are the Market growth Limitations?
Growth restrictions come from the high rate of reinterventions. These reoperations increase costs and inconveniences for patients.
How much is the interspinous market growing?
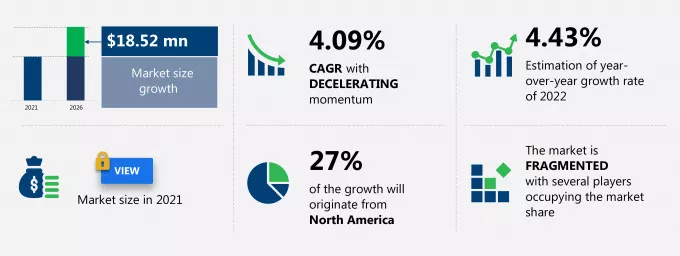
- Interspinous device´s global market size was US$ 70.94 Mn in 2.021.
- The TMR study estimates it to rise at a CAGR of 6.09% from 2022 to 2031. The global interspinous spacers market is predicted to value over US$ 127.33 Mn by 2031.
- The Technavio study estimates a lower growth rate with CAGR of 4.09%.
- The US will particularly grow during the forecast period. In 2021, they held the highest share of this market (27%), driven by the high incidence of spinal stenosis, degenerative disc conditions, and the beneficial reimbursement policies for interspinous spacer devices.
Which are the competitors and products?
We have incorporated more than 60 devices in our interspinous section even though some of them are no longer on the market: https://thespinemarketgroup.com/category/interspinous/:
List of the relevant Interspinous devices:
- Rocker (BIOMECH)
- Giglio Interspinous Fusion System (TSUNAMI MEDICAL)
- U-Device Interspinous Stabilisation Device (NORMMED)
- InsEx (LfC)
- AERIAL™ Interspinous Fixation (Globus Medical)
- Alpine XC Adjustable MIS Fusion System (Zimvie)
- Axle (Xtant Medical)
- AILERON (Life Spine)
- AILERON-TRX (Life Spine)
- Aspen® MIS Fusion System (ZimVie)
- Affix II Mini (Nuvasive)
- Aperius (Medtronic)
- Benefix™ Interspinous Fixation System (Innosys)
- BacJac (Surgalign) Out of the market
- BridgePoint (ATEC Spine) Out of the market
- BacFuse (Surgalign)
- Clutch® Interspinous Process Device (Spinal Elements)
- Coflex F (Surgalign)
- Coflex Interlaminar (Surgalign)
- DynaZIP (Aurora Spine)
- DIAM (Medtronic)
- DIAM™ Spinal Stabilization System (Companion Spine)
- Evo (Clover Orthopedics)
- Ellipse (Sintea Plustek) Out of the market
- Fixpino (Huvexel)
- Falena (Mikai) Out of the market
- FLEXIS (H.P.I. Medical) Out of the market
- Gelfix (Replication Medical)
- HUVEX (Dio Medical)
- Helifix (ATEC Spine) Out of the market
- HEKTOR (BMK Korea)
- IMPALA® (SIGNUS)
- iFIX® (Interspinous Fusion System) (SeohanCare)
- iSPACE (Elite Surgical)
- ip|spine (Ackermann Medical)
- IntraSPINE® (Cousin Surgery)
- Inspan VEGA with self locking screw fixation (KICVentures Group)
- InterBRIDGE®(Zimmer Biomet.LDR) Out of the market
- Inspan SLIM (KICVentures Group)
- InSWing (Orthofix) Out of the market
- InSpace (Synthes) Out of the market
- KeyLift™ (FloSpine)
- LimiFlex (Simpirica Spine)
- Lobster Project (Diametros Medical)
- Promise (BIOMECH) Out of the market
- Promise-F (BIOMECH) Out of the market
- PITBULL(BMK Korea)
- PrimaLOK (Wenzel Spine)
- QFusion Interspinous Fusion (Techlamed)
- Renegade™ (Globus Medical)
- Romeo2 PAD (Spineart)
- Reli™ SP Spinous (Precision Spine) Out of the market
- Reli™ SP PLUS Spinous Plating System (Precision Spine)
- SPEAR® (Interspinous Stabilization System) (SeohanCare)
- Spinous Process Fixation System (Seaspine) Out of the market
- Spinous Bridge (TST) Out of the market
- Stenofix (Synthes) Out of the market
- SP-FLEX™ (Globus Medical)
- Superion® (Boston Scientific)
- SP-Fix (Globus Medical)
- Spire (Medtronic)
- StabiLink® MIS (Southern Spine)
- Spinos (Privalop) Out of the market
- Swift (AMMTEC)
- TieGER™ Interspinous Compressor (ANT Technology) Out of the market
- UniVise™ (Stryker)
- UniWallis™ System Advances Posterior Dynamic Stabilization (Zimmer Biomet) Out of the market
- UniLink (Zimmer Biomet) Out of the market
- VIA Spinous Process Fixation (Spineology)
- WELLEX™ (Eden Spine) Out of the market
- X-StopPK® (Medtronic) Out of the market
- ZIP 51 (Aurora Spine)
- ZIP LP (Aurora Spine)
- ZIP ULTRA (Aurora Spine)
- Z-Clamp ISP (Spine Up)
We have updated this list including the Brochures, Surgical Techniques and Videos availble. We hope you find it useful and we appreciate and welcome any suggestions or comments.We have updated this list including the available Brochures, Surgical Techniques and Videos. We hope you find it useful and welcome any suggestions or comments. It is possible that we may have missed any device or thought that they are out of the the market. We apologize for this and would appreciate your letting us know so we can correct it.
All video parts, images and documents related to the products are of the sole property of the different companies.All the information is for Educational purpose only! No copyright infringement intended.We encourage you to contact us if you have any comment, suggestion or if you want us to include/remove your videos, images or brochures. Please contact us: [email protected]

Is it true that major health insurers are not covering these procedures.? I read this and was very disappointed. as these appear to be excellent devices to relieve severe back pain without major surgery.
Good morning for your information the Lobster Project 2.0 dynamic interspinous device is not off the market. Diametros Medical Ltd. is its legal manufacturer and the device is MDD certified and in MDR certification process.
Great information! Well done. I’d love to see this analysis and list for 3D printed Ti interbody ACDF devices.
Thank you Mitch! We are already preparing that information! Soon we will publish about 3D printed Cervical cages!