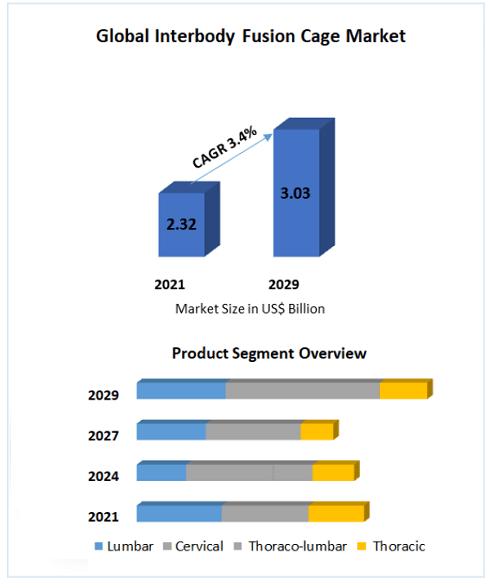
According to Maximize Market Research, the global interbody fusion cage market size reached US$ 2.32 Billion in 2021.It is expected to grow at a CAGR of 3.4% during the forecast period. Global Interbody Fusion Cage Market is expected to reach US$ 3.03 Bn. by 2029..Escalating product demand for decompression of nerves and spine reconstruction during surgeries, expanding geriatric population that is more susceptible to developing spinal cord injuries, and continual technical advancements in the formulation of cage material and design represent some of the key factors driving the market.
Why the stand-alone cages market grows?
- First, because it is a simple procedure: Traditionally, in order to hold an IBD in place, the surgeon had to use a supplemental fixation plate to help. These new stand-alone design by utilizing two screws to help secure the IBD in its functional position, eliminates the need for a supplemental fixation plate making the procedure more easy and fast to implement.
- Second, because the US spinal implant market is a mature market with its largest product segments featuring largely commoditized products and a lack of product innovation.Steady pricing decreases are expected in most segments of the spinal implant market through 2024.Fortunately, shifts in procedure and product mix to premium-priced plate/cage hybrid devices with integrated screw fixation, (stand-alone cages) will help offset declining unit prices.
How many Stand Alone cages compete in this market?
Today we are only going to include the stand-alone cages. Soon we will publish an article about ALIF 3D. To update this list, we have reviewed the portfolios of the different companies counting more than 65 stand-alone anterior lumbar cages that we present below:

- Alicudi-J (TSUNAMI MEDICAL)
- ANTENOR Stand Alone ALIF Cage (OSIMPLANT)
- Esa Alif Peek Cage (NORMMED)
- Idys™-ALIF (CLARIANCE)
- Idys®-ALIF ZP 3DTi (CLARIANCE)
- STALIF M-Ti (CENTINEL SPINE)
- STALIF M FLX (CENTINEL SPINE)
- Redmond ALIF Lumbar Cage (A-SPINE)
- AxTiHA™ (INNOVASIS)
- ASTS-SA (4WEB)
- Ax Stand-Alone ALIF System (INNOVASIS)
- ArcadiusXP L (BRAUN AESCULAP)
- AVS Anchor Lumbar Cage System (STRYKER)
- Arco-SA Ti (NEUROSTRUCTURES)
- Arco-SA (NEUROSTRUCTURES)
- Alif Double Locking Cage A2L (EUROSPINE)
- Aero-AL (STRYKER)
- Axis-ALIF Modular Cage (AXIS SPINE)
- ADVANTAGE ALIF (PRECISION SURGICAL)
- A-Link Z (ACUITY)
- Aurora-Australis ALIF Spinal system (PRISM SURGICAL)
- BIG® ST (SIGNUS)
- Brigade (NUVASIVE)
- Base Interfixated Device (NUVASIVE)
- CORBEL™ Lateral ALIF Interbody Spacer (GLOBUS MEDICAL)
- Chesapeake Anterior-Lumbar (STRYKER)
- Dyna-Link (LIFE SPINE)
- Dyna-Link Elite (LIFE SPINE)
- Durango Stand-Alone (ZIMVIE)
- Dyna-Link Titanium (LIFE SPINE)
- Endoskeleton TAS (MEDTRONIC)
- El Capitan Anterior Lumbar Interbody Fusion System (ASTURA MEDICAL)
- ENZA™-A Titanium Anterior Lumbar Interbody Fusion (CAMBER SPINE)
- Eminent Spine’s ALIF Stand-Alone System (EMINENT SPINE)
- ENZA MIS Zero Profile Anterior Interbody Fusion (CAMBER SPINE)
- InFix (ZIMVIE)
- Irix-A (XTANT MEDICAL)
- Indy Standalone ALIF System (ALTUS SPINE)
- InterPlate L-Ti (PARADIGM BIODEVICES)
- IdentiTiTM ALIF Standalone Interbody System (ATEC SPINE)
- HARRIER™ SA (CHOICE SPINE)
- FBC 921 (FBC)
- Independence MIS™ System (GLOBUS MEDICAL)
- IMPIX ALIF SA™(MEDICREA)
- Fusimax/Saga (VINCULA)
- Leva® AF Anterior Stand-Alone Spacer System (SPINE WAVE)
- HEDRON IA™ 3D Printed Integrated ALIF Spacer (GLOBUS MEDICAL)
- kili Anterior lumbar interbody fusion cage (SPINEWAY)
- JULIET®AN (SPINEART)
- MectaLIF Anterior Interbody Fusion Device (MEDACTA)
- MAGNIFY®-S (GLOBUS MEDICAL)
- Modulus ALIF (NUVASIVE)
- MONDRIAN™ ALIF Cage System (CTL AMEDICA)
- MONUMENT (GLOBUS MEDICAL)
- MAGNUM+ (SPINAL ELEMENTS)
- M3 Stand-Alone ALIF System (CORELINK)
- OneLIF™ interbody fusion device (NOVAPPROACH SPINE)
- Solar™ ALIF (DEGEN MEDICAL)
- Solus (ALPHATEC SPINE)
- SASCA (SOUTHERN MEDICAL)
- Solitaire™ Anterior Spinal System (ZIMVIE)
- Tesera SA Stand-Alone ALIF (RENOVIS)
- ULTIMA™ A.L.I.F. Cage (LANTERNA MEDICAL)
- Vu a•POD™ Prime NanoMetalene® (SEASPINE)
- Vu aPOD (SEASPINE)
- Vault (PRECISION SPINE)
- Zuma™ System (SEASPINE)
###
We have updated this list with the Brochures, Surgical Techniques, and Videos available. We hope you find it useful and we appreciate and welcome any suggestions or comments. We may have missed some of the information or it may be inaccurate. In that case, we apologize for this. Please contact us and let us know.
All video parts, images, and documents related to the products are the sole property of the different companies. All the information is for Educational purposes only! No copyright infringement intended. We encourage you to contact us if you have any comments or suggestions or if you want us to include/remove your videos, images, or brochures. Please contact us at: [email protected]

Would y’all like a photo of Interplate? It’s the only stand-alone Alif plate + interbody cage? Its also is eligible for both the 22845 and 22853 reimbursement codes. The others are eligible as IBDs ONLY with just CPT 22853 eligibility.
Happy to support your efforts any way that I can.
Best regards,
Mike
[email protected]
Thank you very much for your comment and for following us! We have already replaced the Interplate image and we have changed the content of the product information.We hope you find it fine! Please do not hesitate to contact us if you want us to change something in: [email protected]
Best regards,
John Douglas
You forgot Roi C from ZimVie ( formerly ZimmerBiomet)