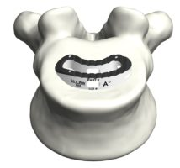
The comprehensive and versatile Tyber Medical TLIF Interbody Spacer was designed for ease of use.
Benefits:
- Optional TyPEEK™ Titanium Plasma Spray:TyPEEK provides an outstanding, new solution for fusion procedures by combining the properties of a Titanium plasma spray with the modulus of PEEK.
- Anatomical Endplate Contact
- Large Graft Window
- PEEK-Optima™ Core
- Simple Instrumentation
- Non-sterile and Sterile Packaging
About TLIF
A transforaminal lumbar interbody fusion (TLIF) is performed to remove a portion of a disc that is the source of back or leg pain. Bone graft is used to fuse the spinal vertebrae after the disc is removed. TLIF provides fusion of the front and back of the lumbar spine. The front portion of the spine, called the anterior column, is stabilized by the interbody spacer and bone graft. The back portion, or posterior column, is locked in place with pedicle screws, rods and additional bone graft, alongside the backs of the vertebra.
About Tyber Medical
Tyber Medical, LLC, Morristown, New Jersey, is focused on addressing an industry-wide need for rapid access to regulatory approved orthopedic implants. These implants are most often used in conjunction with proprietary branded devices produced by companies ranging from small start-ups to large corporations.Tyber Medical produces and markets orthopedic and spinal implants including Cervical Spacers, Anterior Lumbar (ALIF), Posterior Lumbar (PLIF), Transforaminal Lumbar (TLIF), and Direct Lateral (DLIF) Interbody Spacers. http://tybermedical.com