Over the past decade, posterior lumbar interbody fusion (PLIF/TLIF) has evolved—driven in part by the development of expandable interbody cages that adapt in real-time to patient anatomy. These implants have gained popularity among spine surgeons for their ability to minimize neural retraction, improve endplate contact, and restore lumbar alignment, all while integrating seamlessly with minimally invasive techniques.What was once considered novel has now become standard practice in many surgical settings. With in situ expansion capabilities and a growing body of clinical data, expandable cages are redefining the future of PLIF and TLIF procedures.
Why Are Expandable Cages Changing the Game?
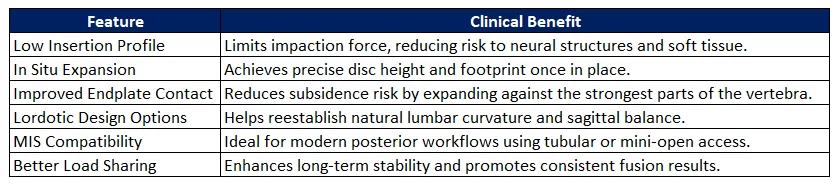
Expandable cages offer key clinical benefits that align closely with the goals of modern PLIF/TLIF procedures. Whether used in traditional open surgeries or through tubular retractors in minimally invasive setups, these devices help reduce surgical trauma while enhancing anatomical restoration. These advantages make expandable cages not only a technological upgrade but also a clinical one—enabling better outcomes with less disruption.
Features &Benefits:
A Growing Field: Nearly 60 Devices Worldwide!
Today’s market includes nearly 60 expandable cages designed for posterior lumbar interbody fusion—a clear sign of the technology’s clinical appeal. With so many systems available, ranging from low-profile titanium cages to 3D-printed constructs with advanced expansion mechanisms, choosing the right implant is increasingly complex.To offer a full picture of the competitive landscape, here is a comprehensive list of expandable PLIF/TLIF cages currently on the market: Full List of Expandable PLIF/TLIF Cages
If We Had to Choose an Implant, Which Ones Would Be the Best?
Technology has advanced to the point where most expandable lumbar cages are of very high quality. Choosing the right one depends on several important factors, which we consider when making our selection. These include the materials used, compatibility with minimally invasive surgery (MIS), the type of expansion mechanism, and the amount of clinical experience available for each device.We selected many implants that best meet these criteria. This does not mean other systems, even those with less clinical history, aren’t just as good—or possibly even better—but our list reflects the specific priorities we evaluated.
Evaluation Criteria
- Expansion Mechanism
- Types: Vertical, angular, multidirectional, bilateral, modular.
- Why it matters: It’s important that the implant can adapt to each patient’s specific anatomy and surgical needs.
- Material and Integration
- Includes: Titanium, PEEK, coated surfaces, 3D-printed structures.
- Why it matters: These materials enhance bone bonding, strength, and biocompatibility.
- Surgical Compatibility (especially MIS)
- What to look for: Implants suitable for minimally invasive surgery and easy to insert.
- Why it matters: MIS reduces surgery time, and trauma, and improves recovery.
- Versatility and Use Cases
- Definition: The implant’s ability to address complex cases, multilevel constructs, or spinal deformities.
- Why it matters: Versatility ensures broader clinical application.
- Clinical Validation and Market Adoption
- Includes: Real-world outcomes, surgeon feedback, and regulatory approvals.
- Why it matters: These factors build confidence in the implant’s safety and effectiveness.
Based on These Criteria, Which are the Most Representative Implants?
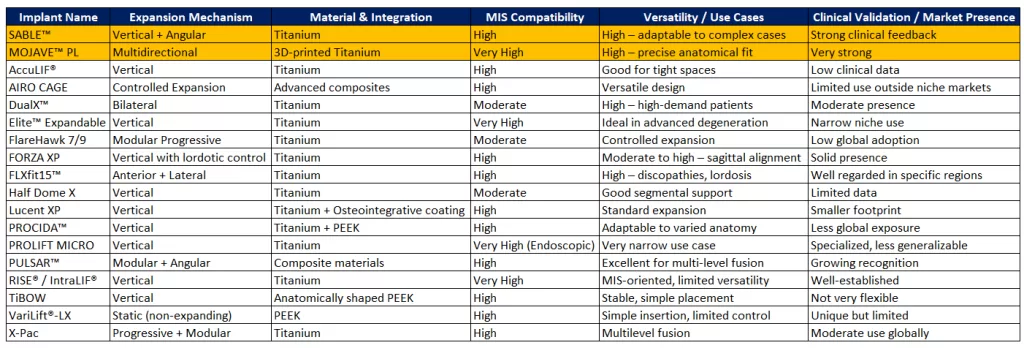
- SABLE™ – Titanium design with lordotic shaping and dual (vertical + angular) expansion for excellent stability and adaptability in complex cases. Clinically reliable with consistent postoperative outcomes.
- MOJAVE™ PL – A 3D-printed, multidirectional system enabling precise anatomical fit and MIS compatibility. Helps minimize migration risk.
- FORZA XP – Designed to restore disc height and sagittal alignment. Ideal for mild to moderate deformities with strong biomechanical support.
- FLXfit15™ – Offers anterior and lateral expansion, with effective lordotic control for treating varied disc pathologies.
- DualX™ – Bilateral expansion enhances segmental stability, especially for high-demand or complex patients.
- PULSAR™ – Modular with angular adjustment, ideal for multilevel fusions using a single, flexible system.
- Elite™ Expandable Fusion System – Compact and suitable for narrow disc spaces, excellent for severe degeneration or tight anatomies.
- RISE® / RISE® IntraLIF® – A trusted system for MIS with great handling and a strong clinical track record.
- X-Pac – Modular and progressively expandable, particularly suited for multilevel posterior fusions.
- PROCIDA™ – Titanium-PEEK hybrid provides a balance between rigidity and adaptability for varying anatomies.
- Half Dome X – Innovative biomechanical design with easy insertion. Limited adoption and clinical history to date.
- FlareHawk7™ / FlareHawk9™ – Modular expansion allows controlled deployment. Adoption is limited by the learning curve and awareness.
- AccuLIF® Expandable Lumbar – Low-profile, MIS-friendly design with vertical expansion. Still lacks long-term clinical data.
- AIRO CAGE – Modular and osteointegration materials, but limited global use and supporting literature.
- VariLift®-LX – Non-expandable post-insertion, simplifying procedures but limiting adaptability.
- Lucent XP Expandable – Titanium cage with bone integration coating. Market presence and data still developing.
- TiBOW Expandable Spacer System – Vertical expansion with anatomical design. Lacks multidirectional capability.
- PROLIFT MICRO Endoscopic Expandable – Compact and optimized for ultra-MIS endoscopic access. Use is limited to experienced centers.
Of all the implants, which one can be considered the most complete?

Both SABLE™ (Globus Medical) and MOJAVE™ PL (Stryker.Now VB Spine)stand out as the most complete implants in their category. Each offers a comprehensive set of features that address the key demands of modern spinal surgery.
- SABLE™ is highly regarded for its mechanical strength, angular expansion, and reliability in complex anatomical situations.
- MOJAVE™ PL, meanwhile, brings cutting-edge advantages in MIS compatibility, multidirectional expansion, and 3D-printed design for enhanced bone integration.
Rather than declaring a single winner, it is more accurate to recognize that these two systems represent the most complete solutions available today. Their strengths complement different surgical needs, and their proven performance makes them the top choices in advanced spinal care.
###
All video parts, images and documents related to the products are of the sole property of the different companies.All the information is for Educational purpose only! No copyright infringement intended.We encourage you to contact us if you have any comment, suggestion or if you want us to include/remove your videos, images or brochures. Please contact us: spinemarketgroup@gmail.com
