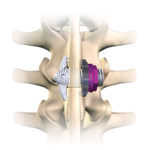
The degenerative stenosis of the lumbar spinal canal is a pathologic disease which leads to the progressive closure of the vertebral foramen and / or the conjugate foramen. Lobster Project is an implantable device for percutaneous interspinous distraction. Lobster Project VIDEO ANIMATION Benefits Lobster Project device stops the segmental extension and it is used to … [Read more...] about Lobster Project
Interspinous
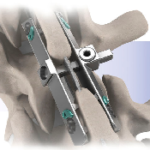
PrimaLOK™SP
PrimaLOK™SP is a next generation posterior lumbar spinous process fixation device, which uniquely compliments VariLift®LX when patient anatomy requires additional stability. Features: About Wenzel Spine Wenzel Spine, Inc. is a medical technology company focused on providing minimally invasive solutions for the treatment of spinal disorders. Headquartered in Austin, … [Read more...] about PrimaLOK™SP
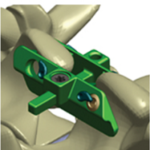
Minuteman®G3R
The Minuteman® G3R is a minimally invasive, interspinous-interlaminar fusion device intended for the temporary fixation of the thoracic, lumbar and sacral spine while awaiting bony fusion to occur. It is designed for attachment to the posterior non-cervical spine at the spinous processes through its bilateral locking plates, and it is intended for use with bone graft material … [Read more...] about Minuteman®G3R
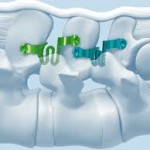
PITBULL
The PITBULL is a posterior, non-pedicle supplemental fixation device intended for use with an interbody cage as an adjunct to fusion at a single level in the lumbar spine (L1~S1).It is intended for attachment to the spinous processes for the purpose of achieving stabilization to promote fusion in patients with degenerative disc disease - defined as back pain of discogenic … [Read more...] about PITBULL
QFusion Interspinous Fusion and Stabilization System
The QFusion Interspinous Fusion and Stabilization System is a percutaneous implantable device that is placed between the spinous bones of the spine. The device consists of a quadrangular body with four wings placed two by two on the opposite ends, which, through the compression of the central body, are opened lifting and approaching simultaneously, until they come into contact … [Read more...] about QFusion Interspinous Fusion and Stabilization System
Renegade™
The Renegade instrumentation was designed for a minimally invasive technique with the use of a guide wire and radiolucent dilation ports.RENEGADE™ is an interspinous distraction device delivered through a minimally invasive lateral approach sparing both tissue and ligaments. Benefits: RENEGADE™ incorporates the benefits of a PEEK spacer with titanium rings for clear … [Read more...] about Renegade™
Romeo2 PAD
Romeo2 PAD is an innovative fixation method that allows posterior fusion while reducing operative time and muscle trauma. Romeo2 PAD VIDEO ANIMATION Benefits: Central peek cage :The central PEEK cage includes a graft window and is designed to promote the fusion process. Rigid interspinous fixation:This innovative fixation method allows posterior fusion while reducing … [Read more...] about Romeo2 PAD
Reli™ SP Spinous
Reli™ SP Spinous Plating System is a posterior, non-pedicle supplemental fixation device, intended for use in the non-cervical spine (T1-S1) of skeletally mature patients to facilitate fusion. It is intended for single level plate fixation/attachment to spinous process for the purpose of achieving supplemental fusion in the following conditions: degenerative disc disease … [Read more...] about Reli™ SP Spinous
Reli™ SP PLUS Spinous Plating System
The Reli™ SP PLUS Spinous Plating System is the first spinous plating system to receive 510(k) marketing clearance from the U.S. Food and Drug Administration (FDA) for utilization with supplemental screw fixation. The two-plate design features an unprecedented fixation option that permits surgeons to attach the plates to the spinous process with screws, in addition to cleats, … [Read more...] about Reli™ SP PLUS Spinous Plating System
SPEAR® (Interspinous Stabilization System)
SPEAR® is an Interspinous Stabilization System manufactured by SeohanCare. Features Easy Implantation MIS by lateral approach Prevention of hyperflexion Reduce subsidence and spinous fractures About Interspinous Devices Interspinous spacers sometimes also called as Interspinous process decompression systems, are the devices implanted between … [Read more...] about SPEAR® (Interspinous Stabilization System)
Spinous Process Fixation System
Our Spinous Process system provides a minimally invasive alternative to pedicle screw fixation to promote fusion and limit spinal extension in the thoracolumbar spine. The system streamlines spinous process fixation, using minimal instruments and straightforward implants. Features Multiple spacer widths to accommodate various patient anatomies Aggressive spikes for … [Read more...] about Spinous Process Fixation System
Spinous Bridge
Spinous Bridge is both an interspinous and interlaminar device that provides segmental stabilization; controlling motion rather than providing fusion. Spinous Bridge is indicated for spinal stabilization after surgically addressing neural compression from soft and bony stenosis of the spinal cord. The interlaminar stabilization provided by Spinous Bridge is ideal for facet … [Read more...] about Spinous Bridge
Rocker
Rocker Interspinous Process System is a decompression spacer that is used to relieve pain from degenerative disc disease. With its unique mechanical design, placing and matching between interspinous process and interlamina to increase the support stability. Rocker VIDEO ANIMATION Rocker VIDEO ANIMATION Extended Rocker Brochure.pdf Features: Anatomic H-shape … [Read more...] about Rocker
SP-FLEX™
SP-FLEX™ is viscoelastic interspinous device designed to be placed between adjacent spinous processes to indirectly decompress lumbosacral spinal segments by maintaining distraction and to restore foraminal height while stabilizing the spinal segment with the attached polyethylene terephthalate (PET) bands.SP-FLEX™ is a polycarbonate urethane (PCU) based interspinous … [Read more...] about SP-FLEX™
StabiLink® MIS
StabiLink™ MIS Spinal Fixation System that has recently received its FDA clearance and CE Mark is designed to provide spinal fixation in lumbar fusion procedures, including the treatment of degenerative disc disease, spinal tumors and trauma.The principle benefit of the patented StabiLink® MIS Spinal Fixation System is that it is placed between the spinous processes, away from … [Read more...] about StabiLink® MIS
Superion®
Superion® Interspinous Spacer (ISS) is a motion-preserving, indirect decompression spinal implant system for the treatment of moderate lumbar spinal stenosis. Superion is the most advanced and least invasive ISS available or in development. Superion VIDEO ANIMATION Superion.SGT-Vertiflex.pdf Benefits: • One piece, expandable implant• Minimally invasive midline approach• … [Read more...] about Superion®
Stenofix
StenoFix is an interspinous implant used after decompressive surgery to preserve the foraminal height and to offload the posterior spinal elements. Stenofix-SGT.DePuySynthes (Synthes).pdf Benefits: - W-shaped spring allows dampening of high axial loads - Ease of use Divergent wings ensure an easy implant insertion even over large spinous processes Wings do not need … [Read more...] about Stenofix
Spinos
Spinos by privelop is an innovative implant for use in the treatment of low back pain. It enables the distance between the spinous processes to be maintained, while preserving full mobility.The in-situ continuous adjustability of the interspinous distance affords dynamic stabilisation of the spinal motion segment, whilst effectively preventing narrowing of the neural foramina … [Read more...] about Spinos
SP-Fix
SP-Fix is a spinous process fixation device that is designed to provide structural stability, indirect decompression and immobilization of the spinous processes of adjacent vertebrae. SP-Fix VIDEO ANIMATION SP-Fix® is implanted using a minimally invasive approach and preserves the supraspinous ligament. SP-Fix® offers surgeons an easy-to-use system that helps preserve … [Read more...] about SP-Fix