PARIS (France), BOULDER (CO, USA), July 17, 2024, - 6:00 pm CEST - SpineGuard (FR0011464452 – ALSGD), an innovative company that deploys its DSG® (Dynamic Surgical Guidance) local conductivity sensing technology to secure and streamline the placement of bone implants,today announces the filing of its “510K” regulatory dossier in the US with the FDA, seeking clearance to … [Read more...] about SpineGuard Files Its “510K” Dossier in the US for the Clearance of Its New Smart Drilling Device Dedicated to Sacroiliac Joint Fusion
2024
Globus Medical Receives FDA 510(k) Clearance for ExcelsiusFlex™ and ACTIFY™ 3D Total Knee System
AUDUBON, Pa., July 17, 2024 (GLOBE NEWSWIRE) -- Globus Medical, Inc. (NYSE: GMED), a leading musculoskeletal solutions company, today announced it recently received 510(k) clearance by the U.S. Food and Drug Administration (FDA) for ExcelsiusFlex™ with Total Knee Arthroplasty (TKA) application. This new robotic navigation platform joins the already … [Read more...] about Globus Medical Receives FDA 510(k) Clearance for ExcelsiusFlex™ and ACTIFY™ 3D Total Knee System
DiscGenics Announces US FDA Approval to Proceed with Phase III Clinical Evaluation of Allogeneic, Injectable Disc Progenitor Cell Therapy for Symptomatic Lumbar Degenerative Disc Disease
SALT LAKE CITY, July 16, 2024 /PRNewswire/ -- DiscGenics, Inc., a privately held, late-stage clinical, biopharmaceutical company developing allogeneic, cell-based, regenerative therapies for musculoskeletal degeneration, today announced it has gained acceptance from the U.S. Food and Drug Administration (FDA) for the clinical protocols and Chemistry, Manufacturing, and Controls … [Read more...] about DiscGenics Announces US FDA Approval to Proceed with Phase III Clinical Evaluation of Allogeneic, Injectable Disc Progenitor Cell Therapy for Symptomatic Lumbar Degenerative Disc Disease
SMAIO Announces Its First-half 2024 Sales
July 16, 2024 12:00 PM Eastern Daylight Time LYON, France--(BUSINESS WIRE)--Regulatory News: SMAIO (Software, Machines and Adaptative Implants in Orthopaedics – Euronext Growth Paris, ISIN: FR0014005I80 / Ticker: ALSMA), a French player specialized in complex spine surgery with a global offer comprising software, adaptative implants and related … [Read more...] about SMAIO Announces Its First-half 2024 Sales
Orthofix Names Stephanie Walsh Chief Human Resources Officer
LEWISVILLE, Texas--(BUSINESS WIRE)--Orthofix Medical Inc. (NASDAQ:OFIX), a leading global spine and orthopedics company, today announced the appointment of Stephanie Walsh as Chief Human Resources Officer. A seasoned human resources executive, Walsh joins Orthofix from ResMed Inc., a San Diego-based medical device company, where she served most recently as Vice President of … [Read more...] about Orthofix Names Stephanie Walsh Chief Human Resources Officer
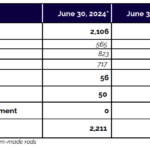
SpineGuard announces its H1 2024 revenue
PARIS (France), BOULDER (CO, USA), July 15, 2024, - 6:00 pm CEST - SpineGuard (FR0011464452 – ALSGD), an innovative company that deploys its DSG® (Dynamic Surgical Guidance) local conductivity sensing technology to secure and streamline the placement of bone implants, today announced its first half 2024 revenue. Pierre Jérôme, Co-founder, Chairman and CEO of SpineGuard, … [Read more...] about SpineGuard announces its H1 2024 revenue
Is Medtronic An Investment Opportunity?
In the investment world, financial media often focus on tech giants like Apple and Microsoft, overshadowing other equally valuable companies. One such hidden gem is Medtronic (MDT), a leading company in the healthcare products sector with over seventy years of history and a market capitalization nearing $100 billion. Despite not capturing as many headlines as the big tech … [Read more...] about Is Medtronic An Investment Opportunity?
Landmark Clinical Study Demonstrates Superiority of 3-Level Circumferential Cervical Fusion Over Anterior Cervical Fusion Alone
PLEASANTON, Calif., July 11, 2024 /PRNewswire/ -- Providence Medical Technology announces FDA Clearance of its CORUS™ Posterior Cervical Stabilization System (PCSS) for the treatment of up to 3-level cervical Degenerative Disc Disease (DDD). The FDA clearance was based on results from the FUSE IDE study, a prospective, multicenter, randomized controlled trial … [Read more...] about Landmark Clinical Study Demonstrates Superiority of 3-Level Circumferential Cervical Fusion Over Anterior Cervical Fusion Alone
NanoHive Medical and DirectSync Surgical Unveil New Hive Lumbar Cage with Piezo Smart Sensor
In recent years, the field of spinal implants has witnessed significant advancements, particularly with the integration of intelligent technologies. These innovations are transforming spinal surgeries, enhancing patient outcomes, and reducing healthcare costs. In October of last year, the strategic collaboration between NanoHive Medical and DirectSync Surgical was announced, … [Read more...] about NanoHive Medical and DirectSync Surgical Unveil New Hive Lumbar Cage with Piezo Smart Sensor
DiscGenics Announces the International Journal of Spine Surgery Has Published Results from an FDA-Approved Study of an Allogeneic Disc Progenitor Cell Therapy for the Treatment of Adults with Lumbar Disc Degeneration
SALT LAKE CITY, July 9, 2024 /PRNewswire/ -- DiscGenics, Inc., a privately held, late-stage clinical, biopharmaceutical company developing allogeneic, cell-based, regenerative therapies for musculoskeletal degeneration, today announced publication of results in the International Journal of Spine Surgery from the combined Phase I/Phase II, first-in-human clinical study of an … [Read more...] about DiscGenics Announces the International Journal of Spine Surgery Has Published Results from an FDA-Approved Study of an Allogeneic Disc Progenitor Cell Therapy for the Treatment of Adults with Lumbar Disc Degeneration
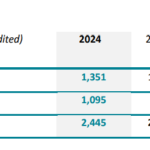
IMPLANET Reports Revenue of €4.1 Million in the First Half of 2024
BORDEAUX, France & BOSTON--(BUSINESS WIRE)--Regulatory News:IMPLANET (Euronext Growth: ALIMP, FR0013470168, eligible for PEA-PME equity savings plans), a medical technology company specializing in implants for orthopedic surgery and the distribution of technological medical equipment, today announces its revenue for the first half of 2024. Ludovic Lastennet, … [Read more...] about IMPLANET Reports Revenue of €4.1 Million in the First Half of 2024
(Updated 2024): +20 Facet Devices to Know..!
Today, for the first time we want to feature a compilation of facet devices. We have also included some that are no longer on the market. Many of you ask us to include some old ones with their corresponding brochures in case of withdrawal or revision. We already know that facet implants are not the most popular or attractive implants, but in any case, they are another surgical … [Read more...] about (Updated 2024): +20 Facet Devices to Know..!
Spineway continues its sales and marketing efforts in Europe
The Spineway Group, a specialist in innovative implants for the treatment of severe spine disorders, announces that it participated in the 38th edition of GEER, the annual congress of the Spanish Spine Society, held in Malaga in May. GEER is the annual meeting of spine surgeons in Spain, like the SFCR Congress held in Montpellier at the beginning of June.1 Spineway’s … [Read more...] about Spineway continues its sales and marketing efforts in Europe
PRODORTH Transports Its Products to the Future with AR Technology!
At PRODORTH , we are taking our commitment to innovation and technology a step further by showcasing our products in Augmented Reality (AR) format. This innovative approach allows our customers to examine our products in detail from every angle and experience how they would look in real-world environments. AR technology makes the complex details and functionality … [Read more...] about PRODORTH Transports Its Products to the Future with AR Technology!
SpineCraft and Spartan Medical Announce Strategic Partnership
CHICAGO and ROCKVILLE, Md., July 2, 2024 /PRNewswire/ -- SpineCraft, a leading spinal surgery implants and instruments developer and manufacturer, announces a strategic partnership with Spartan Medical Inc., a veteran-owned and operated medical solutions company, focused on providing innovative products to the Department of Veterans Affairs (VA), … [Read more...] about SpineCraft and Spartan Medical Announce Strategic Partnership
Epica International, Inc. Celebrates FDA 510(k) Clearance for Revolutionary See Factor CT3™
LANDRUM, S.C., June 28, 2024 (Newswire.com) - Epica International, a leader in advanced imaging solutions and industrial robotics, is thrilled to announce that its cutting-edge See Factor CT3™ System has received an updated 510(k) clearance from the U.S. Food and Drug Administration (FDA). This landmark achievement underscores Epica International’s commitment to … [Read more...] about Epica International, Inc. Celebrates FDA 510(k) Clearance for Revolutionary See Factor CT3™
Spineart Appoints Laurent Nodé-Langlois as Chief Technology Officer to Lead Enabling Technologies Business Unit
GENEVA, June 28, 2024 /PRNewswire/ -- Spineart, a fast-growing company specialized in spine surgery innovation, is pleased to announce the appointment of Laurent Nodé-Langlois as Chief Technology Officer. In his new role, Laurent will be based in the United States and will lead Spineart's strategic initiatives in enabling technologies, focusing on … [Read more...] about Spineart Appoints Laurent Nodé-Langlois as Chief Technology Officer to Lead Enabling Technologies Business Unit
Advanced technology from DePuy Synthes: ALTALYNE™ Ultra Alignment System for Complex Spine
Scoliosis is an abnormal curvature of the spine, and adolescent idiopathic scoliosis (AIS) is the most common spinal deformity in the pediatric population, with worldwide prevalence ranging from 0.47 percent to 12 percent. Surgical treatment of AIS traditionally involves three-dimensional correction with rods and fusion of the spine. A significant challenge associated with the … [Read more...] about Advanced technology from DePuy Synthes: ALTALYNE™ Ultra Alignment System for Complex Spine
Medtronic announces departure of Karen Parkhill, Chief Financial Officer
DUBLIN, June 26, 2024 /PRNewswire(opens new window)/ -- Medtronic plc (NYSE: MDT), a global leader in healthcare technology, today announced that Karen Parkhill will resign as executive vice president and chief financial officer to accept the role of chief financial officer for HP Inc. "On behalf of our employees, our executive committee and our board of directors, I want to … [Read more...] about Medtronic announces departure of Karen Parkhill, Chief Financial Officer