ATLANTA, July 31, 2024 /PRNewswire/ -- MiRus has received Breakthrough Device Designation from the FDA for the EUROPA®Posterior Cervical System, based on it's proprietary rhenium alloys, for treatment of the cervical and upper thoracic spine. The EUROPA® PCF system is built around a 2.9 mm MoRe rod which is much smaller than current commercial … [Read more...] about MiRus Receives Breakthrough Device Designation for Spine Implant
2024
Stryker reports second quarter 2024 operating results
Portage, Michigan, July 30, 2024 (GLOBE NEWSWIRE) -- Stryker (NYSE:SYK) reported operating results for the second quarter of 2024: Second Quarter Results "We delivered another quarter of strong organic sales growth," said Kevin A. Lobo, Chair and CEO. "We remain committed to our margin expansion goals and are excited about our product and M&A … [Read more...] about Stryker reports second quarter 2024 operating results
ATEC Reports Second Quarter 2024 Financial Results And Raises Full-Year Guidance
CARLSBAD, Calif.--(BUSINESS WIRE)-- Alphatec Holdings, Inc. (NASDAQ:ATEC), a provider of innovative solutions dedicated to revolutionizing the approach to spine surgery, today announced financial results for the quarter ended June 30, 2024, and recent corporate highlights. Recent Highlights “In the second quarter, ATEC’s procedural thesis perpetuated best-in-class top … [Read more...] about ATEC Reports Second Quarter 2024 Financial Results And Raises Full-Year Guidance
Life Spine Announces FDA 510(k) Clearance for the ARx® SAI Spinal Fixation System
HUNTLEY, Ill.--(BUSINESS WIRE)--Life Spine, a medical device company that designs, develops, manufactures and markets products for the surgical treatment of spinal disorders, announced today that it has received clearance from the U.S. Food & Drug Administration (FDA) to market the ARx SAI (Sacral Alar Iliac) Spinal Fixation System. The ARx SAI Spinal Fixation System … [Read more...] about Life Spine Announces FDA 510(k) Clearance for the ARx® SAI Spinal Fixation System
SURGLASSES Announces Successful First Clinical Implementation of Caduceus S AR Surgical Navigation System in Thailand
ONTARIO, Calif., July 31, 2024 /PRNewswire/ -- Leading augmented reality (AR) medical technology company SURGLASSES today announced that, in collaboration with exclusive distributor Goodlifeintertrade.co. Ltd., it has successfully registered and clinically implemented its AR-based Caduceus S surgical navigation system in Thailand. This groundbreaking system is … [Read more...] about SURGLASSES Announces Successful First Clinical Implementation of Caduceus S AR Surgical Navigation System in Thailand
Stryker’s Spine Guidance 5 Software featuring Copilot receives 510(k) clearance from FDA
LEESBURG, Va. , July 30, 2024 /PRNewswire/ -- Stryker (NYSE:SYK), a global leader in medical technologies, today announced that its Q Guidance System with Spine Guidance 5 Software featuring Copilot received 510(k) clearance from the U.S. Food and Drug Administration. This first-to-market technology seamlessly integrates smart-powered* instruments into Stryker's growing … [Read more...] about Stryker’s Spine Guidance 5 Software featuring Copilot receives 510(k) clearance from FDA
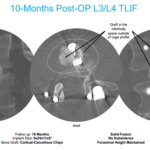
Kleiner Device Labs Releases Scan of 10-Months Post-Surgery Utilizing KG2 Surge Flow-Thru Interbody System
July 29, 2024 -- Incline Village, Nev., -- “This patient scan 10 months post-surgery utilizing the KG®2 Surge® for a TLIF procedure shows obvious fusion, and demonstrates the value of a biologic foundation of graft between vertebral end plates, maintaining disc height, preventing implant subsidence and preservation of foraminal height”, said Jeff Kleiner, MD, founder and CEO of … [Read more...] about Kleiner Device Labs Releases Scan of 10-Months Post-Surgery Utilizing KG2 Surge Flow-Thru Interbody System
Safe – First-Half 2024 Sales
Fleurieux-sur-l’Arbresle - France - July 29, 2024, 6.00 p.m. The group Safe (FR001400F1V2 – ALSAF) announces consolidated sales for 1st half 2024. The Safe Group's consolidated sales for the 1st half of 2024 came to €2.6 million, maintaining a good level. Over the period, the new management team implemented a large-scale plan to transform the Group. Strategic decisions have … [Read more...] about Safe – First-Half 2024 Sales
Captiva Spine Announces First Navigated Office-based SI Fusion Procedure Using Watchtower Roam Spine Navigation System and Transfastern-PSF Posterior SI-Fusion System
Plano, TX – July 2024—Neurosurgeon Dr. Abdul Baker, MD, FAANS, FACS, FCNS, and Captiva Spine has set a new benchmark in MIS spine surgery by performing their first navigated, office-based sacroiliac (SI) joint fusion using conscious sedation (patient awake). The procedure utilized Captiva Spine’s advanced WatchTower® ROAM™ Spine Navigation System and TransFasten-PSF Posterior … [Read more...] about Captiva Spine Announces First Navigated Office-based SI Fusion Procedure Using Watchtower Roam Spine Navigation System and Transfastern-PSF Posterior SI-Fusion System
Providence St. Joseph Hospital Performs World’s First Spine Surgery Using eCential Robotics’ Open Spine Robot, with Spineart Compatible Implants
ORANGE, Calif. July 25, 2024 – Providence St. Joseph Hospital announced it has successfully performed the world’s first robotic spinal surgery using a new open spine platform by eCential Robotics to implant a Spineart medical device. eCential Robotics, a surgical robotics company which developed the open surgical platform and Spineart, a leader in spine surgery innovation, … [Read more...] about Providence St. Joseph Hospital Performs World’s First Spine Surgery Using eCential Robotics’ Open Spine Robot, with Spineart Compatible Implants
Spineway a Gold Sponsor at the SILACO Congress in Mexico – Organization of a special event with Latin American distributors
Ecully, July 24, 2024 – The Spineway Group, a specialist in innovative implants for the treatment of severe spine disorders, is taking part in the Sociedad Iberolatinoamericana de Columna (SILACO) Congress in Mexico from July 24 to 27, 2024 with two local partners, Spinemed and Mava. As a Gold Sponsor of this major event in Latin America, Spineway will increase its visibility … [Read more...] about Spineway a Gold Sponsor at the SILACO Congress in Mexico – Organization of a special event with Latin American distributors
LEM Surgical Announces Core Patent for Bilateral Robotic System
BERN, SWITZERLAND, July 23, 2024 /EINPresswire.com/ -- LEM Surgical AG, a leader in surgical robotics, proudly announced the publication of one of their core patents for a Bilateral Surgical Robotic System. This innovative system is poised to revolutionize the field of robotic spinal surgery, offering unprecedented precision and efficiency for complex surgical … [Read more...] about LEM Surgical Announces Core Patent for Bilateral Robotic System
Biogennix Launches Agilon Surgical Matrix
IRVINE, Calif. — July 24, 2024 — Biogennix, an osteobiologics company specializing in innovative healing solutions for surgical procedures, has announced the launch of its new Agilon Surgical Matrix wound care product. Agilon Surgical Matrix is a wound care solution derived from 100% bovine Type 1 collagen. Upon contact with a new or open wound, whether surgically or … [Read more...] about Biogennix Launches Agilon Surgical Matrix
ATEC Launches EOS InsightTM, an End-To-End Spine Surgery Platform Powered by EOS Imaging and AI
CARLSBAD, Calif.--(BUSINESS WIRE)--Alphatec Holdings, Inc. (Nasdaq: ATEC), a provider of innovative solutions dedicated to revolutionizing the approach to spine surgery, today announced the commercial launch of EOS Insight, a groundbreaking, end-to-end spine surgery platform powered by standardized EOSedge™ scans and AI. With the first customer implementation complete, the EOS … [Read more...] about ATEC Launches EOS InsightTM, an End-To-End Spine Surgery Platform Powered by EOS Imaging and AI
Camber Spine Receives FDA Clearance for SPIRA-A Integrated Fixation System
KING OF PRUSSIA, PA — July 23, 2024 – Camber Spine, a leading innovator in spine and medical technologies, announced today that it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its SPIRA-A Integrated technology. Part of the SPIRA product platform, the SPIRA-A Integrated Fixation System is an anterior lumbar interbody fusion device that has an … [Read more...] about Camber Spine Receives FDA Clearance for SPIRA-A Integrated Fixation System
Integrated Endoscopy Announces Commercial Release of Nuvis Wireless Camera System for International Markets
Irvine, CA – July 23, 2024 – Integrated Endoscopy, a medical device company pioneering the development of the wireless arthroscopic surgery market, has announced the commercial launch of its advanced Nuvis Wireless Camera System. The Nuvis wireless camera system offers surgeons and health centers next-generation wireless communication at a cost below traditional corded … [Read more...] about Integrated Endoscopy Announces Commercial Release of Nuvis Wireless Camera System for International Markets
eCential Robotics Receives FDA 510(k) Clearance for Spine Navigation and Robotic-Assistance Device
GIÈRES (GRENOBLE), France and NASHVILLE, Tenn., July 22, 2024 /PRNewswire/ -- eCential Robotics is proud to announce that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for an innovative Spine Navigation and Robotic-Assistance Device. This device provides a new, advanced solution for planning and instrumenting spinal … [Read more...] about eCential Robotics Receives FDA 510(k) Clearance for Spine Navigation and Robotic-Assistance Device
Augmented Reality Revolutionizes Spine Surgery with xvision Spine System
Dr. Harshpal Singh of Premier Brain & Spine is one of the first surgeons in the state to perform spine surgery using the Augmedics xvision Spine System, a groundbreaking augmented reality (AR) guidance system. This innovative technology allows surgeons to "see through" a patient's anatomy as if they have "x-ray vision." The xvision Spine System is the first AR guidance … [Read more...] about Augmented Reality Revolutionizes Spine Surgery with xvision Spine System
eCential Robotics Appoints Clément Vidal as New Chief Executive Officer
GIÈRES (GRENOBLE), France and NASHVILLE, Tenn., July 18, 2024 /PRNewswire/ -- eCential Robotics is pleased to announce the appointment of Clément Vidal as its new Chief Executive Officer. Clément Vidal, an Ecole Polytechnique and Stanford University graduate, has 20 years of experience in the Pharmaceutical and Medical Device industry, especially as CEO of surgical robotics … [Read more...] about eCential Robotics Appoints Clément Vidal as New Chief Executive Officer