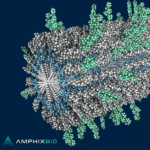
CHICAGO, Oct. 3, 2024 /PRNewswire/ -- Amphix Bio, a company developing a new class of regenerative medicine therapies, announced today it has received a Breakthrough Device designation from the U.S. Food and Drug Administration (FDA) for a drug-device combination product for bone regeneration. The designation covers the use of the therapeutic device to treat … [Read more...] about FDA grants Breakthrough Device designation to Amphix Bio’s bone regeneration product
2024
Globus Medical Launches the Next Wave of Orthopedic Trauma Solutions
AUDUBON, Pa., Oct. 02, 2024 (GLOBE NEWSWIRE) -- Globus Medical, Inc. (NYSE: GMED), a leading musculoskeletal solutions company, announces the continued growth and expansion of its orthopedic trauma product portfolio. Globus has introduced several new system extensions so far in 2024 and received 510(k) clearance from the U.S. Food and Drug Administration for its first … [Read more...] about Globus Medical Launches the Next Wave of Orthopedic Trauma Solutions
SpineGuard obtains FDA clearance for commercial release of its “PsiFGuard” new smart drilling device dedicated to sacroiliac joint fusion
SpineGuard (FR0011464452 - ALSGD), an innovative company that deploys its DSG® (Dynamic Surgical Guidance) local conductivity sensing technology to secure and streamline the placement of bone implants, announced today the authorization under 510K #241895 by the FDA (Food and Drug Administration) to commercialize in the United States its new PsiFGuard device designed … [Read more...] about SpineGuard obtains FDA clearance for commercial release of its “PsiFGuard” new smart drilling device dedicated to sacroiliac joint fusion
Bioreplica: A Revolutionary Spanish Startup Transforming Spinal Prosthetics!
Based in the sunny archipelago of Las Palmas de Gran Canaria, Spain, Bioreplica is making waves with its cutting-edge developments in prosthetic technology. Founded by Rodrigo de Pablos, the company has quickly emerged as a pioneering force in the field of spine care, with its groundbreaking spinal prosthesis already receiving critical acclaim and prestigious awards on both … [Read more...] about Bioreplica: A Revolutionary Spanish Startup Transforming Spinal Prosthetics!
33 Medical Wins 2024 Best Technology in Spine Award for Innovative NuvoDisc® Device
Boca Raton, FL, Sept. 27, 2024 (GLOBE NEWSWIRE) -- 33 Medical, Inc., a leader in cutting-edge spinal health innovations, is proud to announce that its NuvoDisc® medical device has been awarded the prestigious 2024 Best Technology in Spine Award. This accolade recognizes the NuvoDisc® as a revolutionary advancement in the treatment of degenerative disc disease … [Read more...] about 33 Medical Wins 2024 Best Technology in Spine Award for Innovative NuvoDisc® Device
Over 7,500 Procedures Completed in Two Years with Centinel Spine’s prodisc® C Vivo and prodisc C SK Cervical Total Disc Replacement System Since U.S. Launch
WEST CHESTER, Pa., Sept. 26, 2024 /PRNewswire/ -- Centinel Spine®, LLC ("the Company"), the leading global medical device company focused exclusively on treating cervical and lumbar spinal disease with the most complete and clinically-proven total disc replacement (TDR) technology platform in the world (prodisc®), today announced the completion of 7,500 U.S. cases … [Read more...] about Over 7,500 Procedures Completed in Two Years with Centinel Spine’s prodisc® C Vivo and prodisc C SK Cervical Total Disc Replacement System Since U.S. Launch
Synergy Spine Solutions announces completion of patient enrollment in the Synergy Disc® 2-Level IDE clinical trial
LOUISVILLE, Colo., Sept. 25, 2024 /PRNewswire/ -- Synergy Spine Solutions Ltd, an innovative orthopedic medical device developer focused on artificial cervical disc replacement, today announced that it has completed patient enrollment in its U.S. 2-Level IDE clinical trial. Conducted under a U.S. Investigational Device Exemption (IDE), the 2-Level trial will … [Read more...] about Synergy Spine Solutions announces completion of patient enrollment in the Synergy Disc® 2-Level IDE clinical trial
Medtronic expands AiBLE™ spine surgery ecosystem with new technologies and Siemens Healthineers partnership
GALWAY, Ireland and CHICAGO, Sept. 25, 2024 /PRNewswire/ -- Medtronic plc (NYSE: MDT), a global leader in healthcare technology, announced today at the North American Spine Society (NASS) 39th Annual Meeting in Chicago the commercial launch of several software, hardware, and imaging innovations. These enhancements are designed to advance AiBLE™, the Medtronic smart ecosystem of … [Read more...] about Medtronic expands AiBLE™ spine surgery ecosystem with new technologies and Siemens Healthineers partnership
elliquence and Implanet Form Partnership for US Distribution of Ultrasonic Bone Scalpel Olea in Endoscopic Spine Market
Spine tech innovators elliquence and Implanet to exhibit at NASS in Chicago, September 25-28, 2024 (elliquence LLC Booth 2419 Implanet Booth 2412) elliquence LLC, specializing in advanced minimally invasive surgical technologies, and Implanet (Euronext Growth: ALIMP, FR0013470168, eligible for PEA-PME), a medical technology company specializing in implants for … [Read more...] about elliquence and Implanet Form Partnership for US Distribution of Ultrasonic Bone Scalpel Olea in Endoscopic Spine Market
OrtoWay-US Inc. Announces Granting of U.S. Patent for OrtoWell® Distractor System for Spinal Surgery Corpectomy
HORSHAM, Pa., September 23, 2024 (Newswire.com) - OrtoWay-US Inc., inventors and developers of the world’s first hydraulically-powered OrtoWell® Distractor for spinal surgery, announced today the receipt of a new patent from the U.S. Patent Office for its OrtoWell® Distractor system. The patent, numbered US 12,023017 B1, entitled “Apparatus, Methods and Systems for Spine … [Read more...] about OrtoWay-US Inc. Announces Granting of U.S. Patent for OrtoWell® Distractor System for Spinal Surgery Corpectomy
Cerapedics’ i-FACTOR P-15 Peptide Enhanced Bone Graft, a Trusted Choice in Bone Graft Solutions, Now Approved for Expanded Indications for Use
WESTMINSTER, Colo., Sept. 24, 2024 /PRNewswire/ -- Cerapedics Inc., a global, commercial-stage orthopedics company dedicated to redefining the standard of care for bone repair, today announced the U.S. Food and Drug Administration (FDA) approval for an expansion to the indications for use and labeling for i-FACTOR P-15 Peptide Enhanced Bone Graft. With this label … [Read more...] about Cerapedics’ i-FACTOR P-15 Peptide Enhanced Bone Graft, a Trusted Choice in Bone Graft Solutions, Now Approved for Expanded Indications for Use
NGMedical presents its MOVE®-C Cervical Artificial Disc Prosthesis at NASS and Eurospine
NONNWEILER, SAARLAND, GERMANY, September 18, 2024 /EINPresswire.com/ -- NGMedical GmbH, a medical device manufacturer exclusively focused on creating innovative technologies for spinal application, will present its unique articulating viscoelastic cervical artificial disc MOVE®-C as well as further unique spinal solutions at this year’s annual NASS meeting in Chicago and … [Read more...] about NGMedical presents its MOVE®-C Cervical Artificial Disc Prosthesis at NASS and Eurospine
Spineology® Announces Organization Rebrand
ST. PAUL, Minn.--(BUSINESS WIRE)--Spineology Inc., the pioneer of Conform and Expand™ Technology, today unveiled a new brand and visual identity, logo, and product positioning as part of an extensive rebranding initiative. This new brand defines Spineology’s flagship interbody fusion portfolio, OptiMesh®, and establishes it within a category of its own. OptiMesh is the only … [Read more...] about Spineology® Announces Organization Rebrand
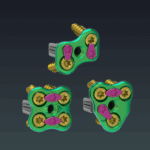
SIGNUS Receives FDA Approval for TETRIS® ST & TETRIS® R ST PLIF Cages
Alzenau, 09/12/2024 – SIGNUS Medizintechnik GmbH is proud to announce the successful FDA approval of TETRIS ® ST and TETRIS ® R ST PLIF cages. These cages, made from structured titanium (ST), combine cutting-edge technology and materials, further expanding the innovative SIGNUS spinal surgery portfolio. SIGNUS is dedicated to continuously developing products with passion and … [Read more...] about SIGNUS Receives FDA Approval for TETRIS® ST & TETRIS® R ST PLIF Cages
SC MEDICA wins 2024 Best Technology in Spine Award for its FFX® Facet FiXation system
Strasbourg, France, September 18, 2024 – SC MEDICA, a leader in innovative spinal solutions, is pleased to announce that it has received the prestigious 2024 Best Technology in Spine Award, organizedby Orthopedics This Week, for its innovative FFX® Facet Fixation system. This award honors the relentless dedication of the engineers, inventors, and medical professionals who … [Read more...] about SC MEDICA wins 2024 Best Technology in Spine Award for its FFX® Facet FiXation system
Nexxt Spine Launches Struxxure® Modular Cervical System
NOBLESVILLE, IN, UNITED STATES, September 18, 2024 /EINPresswire.com/ -- Nexxt Spine, LLC is proud to announce the launch of the Struxxure® Modular Cervical System (MCS). Struxxure MCS is built on more than ten years of proven clinical use of the Struxxure Anterior Cervical Plate system. MCS is designed to offer intraoperative flexibility and modularity, including … [Read more...] about Nexxt Spine Launches Struxxure® Modular Cervical System
eCential Robotics Appoints Experienced Leadership Team to Drive U.S. Market Expansion
NASHVILLE, Tenn., Sept. 19, 2024 /PRNewswire/ -- eCential Robotics, a growth company transforming bone surgery by designing and manufacturing an open robotic and navigation system with unique capabilities, is pleased to announce the addition of Lisa Jacobs and Matthieu Ville to its leadership team. These strategic hires come as part … [Read more...] about eCential Robotics Appoints Experienced Leadership Team to Drive U.S. Market Expansion
Avicenna.AI lands FDA clearance for AI tool that detects cervical spine fracture
La Ciotat, FRANCE – September 19, 2024 – Medical imaging AI company Avicenna.AI today announced that it has received 510(k) clearance from the US Food and Drug Administration for its CINA-CSpine tool. Using a combination of deep learning and machine learning technologies, the company develops AI solutions that automatically detect and prioritize life-threatening … [Read more...] about Avicenna.AI lands FDA clearance for AI tool that detects cervical spine fracture
Amnovis Marks Milestone of 50,000 Implants Delivered with Game-Changing Titanium 3D Printing Process
Aarschot, Belgium – September19, 2024.--Amnovis, a leader in 3D printed titanium implant manufacturing, has reached a significant milestone, delivering over 50,000 titanium implants since 2021 using its groundbreaking, heat treatment-free 3D printing process. This innovative technology is transforming the production of spinal, orthopedic, and cranio-maxillofacial (CMF) … [Read more...] about Amnovis Marks Milestone of 50,000 Implants Delivered with Game-Changing Titanium 3D Printing Process