MIAMI--(BUSINESS WIRE)--H.I.G. Capital (“H.I.G.”), a leading global alternative investment firm with $60 billion of capital under management, is pleased to announce that one of its affiliates has completed the acquisition of the Spine division of ZimVie, Inc (“ZimVie”, NYSE: ZIMV). The acquired business will operate as an independent entity and has been renamed Highridge … [Read more...] about H.I.G. Capital Acquires the Spine Business of ZimVie Rebranded as Highridge Medical
2024
NGMedical Celebrates One-Year Anniversary of the New Headquarter in Germany
NONNWEILER, SAARLAND, GERMANY, March 28, 2024 /EINPresswire.com/ -- NGMedical GmbH, a medical device manufacturer exclusively focused on creating innovative technologies for spinal application is proud to celebrate the one-year anniversary of its state-of-the-art headquarter located in Nonnweiler, Germany. This milestone marks a year of innovation and growth for the … [Read more...] about NGMedical Celebrates One-Year Anniversary of the New Headquarter in Germany
PathKeeper Surgical enters into know-how agreement with Mayo Clinic for low-radiation pediatric spine surgery
KFAR SABA, Israel, March 25, 2024 /PRNewswire/ -- PathKeeper Surgical, an Israeli medical technology company committed to advancing surgical solutions through innovative camera and machine learning technologies, announced it has entered into a know-how agreement with Mayo Clinic to research radiation levels in pediatric spine surgeries. The research will … [Read more...] about PathKeeper Surgical enters into know-how agreement with Mayo Clinic for low-radiation pediatric spine surgery
Centinel Spine® to Sponsor Major Worldwide Live Webinar Symposium on Cervical Disc Arthroplasty
WEST CHESTER, Pa., March 21, 2024 /PRNewswire/ -- Centinel Spine®, LLC ("the Company"), the leading global medical device company focused exclusively on treating cervical and lumbar spinal disease with the most complete and clinically-proven total disc replacement (TDR) technology platform in the world (prodisc®), today announced that it will sponsor an upcoming major … [Read more...] about Centinel Spine® to Sponsor Major Worldwide Live Webinar Symposium on Cervical Disc Arthroplasty
Aurora Spine Corporation Celebrates Second Anniversary of Initial Implantation of the World’s First Bone Density Matched DEXA-C Cervical Interbody Fusion Device
CARLSBAD, Calif., March 20, 2024 (GLOBE NEWSWIRE) -- Aurora Spine Corporation (“Aurora Spine” or the “Company”) (TSXV: ASG) (OTCQB: ASAPF), a manufacturer of innovative spinal implants announced today that it has completed the second full year of implantation of the world’s first patient-bone-density matched interbody device, the cervical interbody DEXA-C. Trent Northcutt, … [Read more...] about Aurora Spine Corporation Celebrates Second Anniversary of Initial Implantation of the World’s First Bone Density Matched DEXA-C Cervical Interbody Fusion Device
Outcomes of a Posterior Approach to SI Joint Fusion with LinQ™ Reflect Clinical Efficacy and Durability at 12 Months
TAMPA, Fla., March 20, 2024 /PRNewswire/ -- Final data from a landmark study on PainTEQ's posterior approach SI joint fusion with the LinQ™ implant revealed improved pain and function scores and an excellent safety profile at 12 months post-operation. The Single Arm, Multicenter, Prospective, Clinical Study on a Minimally Invasive Posterior … [Read more...] about Outcomes of a Posterior Approach to SI Joint Fusion with LinQ™ Reflect Clinical Efficacy and Durability at 12 Months
First Implantation of the MOVE®-C Cervical Artificial Disc Prosthesis from NGMedical GmbH in Mexico
NONNWEILER, SAARLAND, GERMANY, March 19, 2024 /EINPresswire.com/ -- NGMedical GmbH, a medical device manufacturer exclusively focused on creating innovative technologies for spinal application, announces the first implantation of its MOVE®-C cervical artificial disc replacement in Mexico. MOVE®-C combines the features of a second-generation viscoelastic disc prosthesis with … [Read more...] about First Implantation of the MOVE®-C Cervical Artificial Disc Prosthesis from NGMedical GmbH in Mexico
Lucas Vitale Joins Orthofix as Chief People and Business Operations Officer
LEWISVILLE, Texas--(BUSINESS WIRE)-- Orthofix Medical Inc. (NASDAQ:OFIX), a leading global spine and orthopedics company, today announced that Lucas Vitale has joined the company in the newly created role of Chief People and Business Operations Officer. Vitale most recently served as the Chief Human Resources Officer (CHRO) at ReNAgade Therapeutics, a venture backed … [Read more...] about Lucas Vitale Joins Orthofix as Chief People and Business Operations Officer
Carlsmed Raises $52.5M in Series C Financing to Advance Personalized Spine Surgery
CARLSBAD, Calif.--(BUSINESS WIRE)--Carlsmed, an AI-enabled personalized surgery Medtech company, announced today a $52.5M Series C funding round co-led by B Capital and U.S. Venture Partners. These proceeds will be used to accelerate the commercialization of the aprevo® personalized spine surgery platform for lumbar fusion procedures and the development of aprevo® for cervical … [Read more...] about Carlsmed Raises $52.5M in Series C Financing to Advance Personalized Spine Surgery
Spineart Secures more than CHF20 Million in Convertible Financing Following Completion of BAGUERA®C IDE Studies Enrollment
GENEVA, March 18, 2024 /PRNewswire/ -- Spineart, a global spine specialist working with surgeons to accelerate the adoption of cutting-edge technologies, has successfully raised a CHF20 million convertible financing. The funding comes on the heels of the completion of enrollment in the two BAGUERA®C IDE studies, underlining Spineart's commitment to advancing … [Read more...] about Spineart Secures more than CHF20 Million in Convertible Financing Following Completion of BAGUERA®C IDE Studies Enrollment
Eminent Spine’s 3D Titanium Anterior Lumbar Stand-Alone System Usage Report and Clinical Study
Plano, TX, March 14, 2024 --(PR.com)-- Eminent Spine received 510(K) approval of their 3D Titanium Anterior Lumbar Interbody Fusion Stand-Alone System in October of 2022. Since then, Eminent Spine has had a total of 102 3D Titanium ALIF Stand-Alone implants used in procedures in patients who were candidates for an Anterior Lumbar Interbody Fusion. Of these 102 implants, 62 have … [Read more...] about Eminent Spine’s 3D Titanium Anterior Lumbar Stand-Alone System Usage Report and Clinical Study
(UPDATED 2024): +70 Corpectomy Devices to Know..!
Corpectomy systems provide a solution for stabilization of the spine in skeletally mature patients to replace a diseased, collapsed, damaged, or unstable vertebral body due to tumor, osteomyelitis, trauma (i.e. fracture), or for reconstruction following corpectomy performed to achieve decompression of the spinal cord and neural tissues in degenerative disorders. The system is … [Read more...] about (UPDATED 2024): +70 Corpectomy Devices to Know..!
Coverage Momentum Continues for Lumbar Total Disc Replacement as the Top Commercial Payer in Washington Establishes Positive Coverage
WEST CHESTER, Pa., March 12, 2024 /PRNewswire/ -- Centinel Spine®, LLC ("the Company"), the leading global medical device company focused exclusively on treating cervical and lumbar spinal disease with the most complete and clinically-proven total disc replacement (TDR) technology platform in the world (prodisc®), today announced significant expansion of coverage for … [Read more...] about Coverage Momentum Continues for Lumbar Total Disc Replacement as the Top Commercial Payer in Washington Establishes Positive Coverage
Spine Surgery with Apple! First surgery using the Vision Pro in London!
In a groundbreaking development, surgeons in the UK have ushered in a new era of medical innovation by utilizing Apple's cutting-edge virtual reality goggles to conduct a spinal operation for the first time. The pioneering procedure, used at the Cromwell Hospital in London, marks a significant leap forward in surgical technology and has the potential to revolutionize healthcare … [Read more...] about Spine Surgery with Apple! First surgery using the Vision Pro in London!
Spineart Completes Enrollment in the Baguera®C IDE trial for Two-Level Cervical Disc Replacement
GENEVA, March 8, 2024 /PRNewswire/ -- Spineart SA, manufacturer of the BAGUERA®C Cervical Disc Prosthesis, today announced it has completed enrollment in its U.S. IDE trial studying the BAGUERA®C Cervical Disc Prosthesis in patients with cervical disc disease at two contiguous levels between C3 to C7 compared to a commercially marketed cervical disc implant. The … [Read more...] about Spineart Completes Enrollment in the Baguera®C IDE trial for Two-Level Cervical Disc Replacement
Medtronic advances data-driven spine solutions with AI-powered patient reported outcomes
Medtronic, a global leader in medical technology, announced today the launch of the new UNiD™ ePRO service in the United States. This solution will change the way patient outcomes are collected for spine surgeons, reducing the burden on clinic staff and patients. Medtronic has partnered with OBERD, a leading practice intelligence data collection company, to provide spine … [Read more...] about Medtronic advances data-driven spine solutions with AI-powered patient reported outcomes
Xtant Medical Increases Revolving Credit Facility to $17 Million with MidCap Financial
BELGRADE, Mont., March 07, 2024 (GLOBE NEWSWIRE) -- Xtant Medical Holdings, Inc. (NYSE American: XTNT), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today announced that the Company’s revolving credit agreement with MidCap Financial Trust (“MidCap”) was amended to increase the commitment from $8.0 million to $17.0 … [Read more...] about Xtant Medical Increases Revolving Credit Facility to $17 Million with MidCap Financial
SurGenTec® Receives FDA Clearance for OsteoFlo® HydroPutty™, a Hydrophilic Synthetic Bone Graft
BOCA RATON, Fla.--(BUSINESS WIRE)--SurGenTec, a pioneering medical device company specializing in orthopedic and spine technologies, proudly announces the first implantations and 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its revolutionary OsteoFlo HydroPutty Synthetic Bone Graft. OsteoFlo HydroPutty represents a significant advancement in bone … [Read more...] about SurGenTec® Receives FDA Clearance for OsteoFlo® HydroPutty™, a Hydrophilic Synthetic Bone Graft
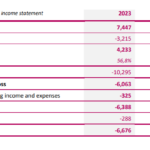
IMPLANET reports its 2023 full-year results
Bordeaux, Boston, March 6, 2024 – 7:30 am CET: IMPLANET (Euronext Growth: ALIMP, FR0013470168, eligible for PEA-PME equity savings plan), a medical technology company specialized in implants for orthopedic surgery and the distribution of technological medical equipment, today announced its 2023 full-year results, as of December 31, 2023, as approved by the Board of Directors on … [Read more...] about IMPLANET reports its 2023 full-year results