The FDA's approval of a spinal implant signifies that the device has undergone a rigorous evaluation to ensure it meets the highest standards of safety, effectiveness, and quality required for use in the United States. This process involves comprehensive preclinical and clinical testing to demonstrate the device's ability to achieve its intended purpose, such as relieving pain … [Read more...] about Innovative Spine Implants and Technologies Approved by the FDA in 2024
2024
The Challenges of CRMs in Spine Sales
In the fast-moving world of spine sales, customer relationships go beyond transactions—they’re partnerships built on trust, knowledge, and long-term commitment. A well-implemented Customer Relationship Management (CRM) system can be a game-changer, but only if it’s tailored to the industry's specific needs. Despite its potential, CRM often presents challenges that can limit the … [Read more...] about The Challenges of CRMs in Spine Sales
Dr. Kingsley R. Chin: Federal Judge Dismisses Money Laundering Charges in His Former Role as CEO of SpineFrontier Inc.
A Massachusetts federal judge has dismissed the money laundering charges brought against Dr. Kingsley R. Chin in his former capacity as CEO of SpineFrontier Inc. BOSTON, Dec. 20, 2024 /PRNewswire/ -- A federal judge has dismissed the money laundering charges brought against Dr. Kingsley R. Chin in his former capacity as CEO of SpineFrontier Inc. … [Read more...] about Dr. Kingsley R. Chin: Federal Judge Dismisses Money Laundering Charges in His Former Role as CEO of SpineFrontier Inc.
Spineway wins new approvals in Vietnam and registers initial orders
Ecully, December 18, 2024 – The Spineway Group, a specialist in innovative implants for the treatment of severe spine disorders, announces that it has obtained approvals for its ACIFBOX, VEOS, KAPHORN and TWIN PEAKS ranges in Vietnam. Following this approval, initial orders have been placed, confirming the growing interest in Spineway products in the region. These approvals … [Read more...] about Spineway wins new approvals in Vietnam and registers initial orders
DuraStat® Launches Commercial Partnership with Medtronic Cranial and Spinal Technologies Business
AUSTIN, Dec. 18, 2024 /PRNewswire/ -- DuraStat LLC, a surgical tissue closure company, has launched an exclusive U.S. commercial partnership with Medtronic's Advanced Energy business within the Cranial & Spinal Technologies Operating Unit. The partnership transforms clinical delivery for the company's flagship DuraStat (spine dural repair) and TissueStat … [Read more...] about DuraStat® Launches Commercial Partnership with Medtronic Cranial and Spinal Technologies Business
Landmark Lumbar Total Disc Replacement Study of Nearly 1,200 Patients Supports Long-term Clinical Success and Durability of Centinel Spine’s prodisc® L System
WEST CHESTER, Pa., Dec. 12, 2024 /PRNewswire/ -- Centinel Spine®, LLC ("the Company"), the leading global medical device company focused exclusively on treating cervical and lumbar spinal disease with the most complete and clinically-proven total disc replacement (TDR) technology platform in the world (prodisc®), today announced the recent release of a landmark study … [Read more...] about Landmark Lumbar Total Disc Replacement Study of Nearly 1,200 Patients Supports Long-term Clinical Success and Durability of Centinel Spine’s prodisc® L System
Augmedics Appoints Paul Ziegler as President and Chief Executive Officer
CHICAGO--(BUSINESS WIRE)--Augmedics, a pioneer in augmented reality (AR) surgical navigation, today announced Paul Ziegler as President and Chief Executive Officer. With the appointment, Gwen Watanabe will transition from interim CEO and serve as Vice Chair of the Board. Ziegler, a 20-year medical device veteran, joins Augmedics with a proven track record in commercial and … [Read more...] about Augmedics Appoints Paul Ziegler as President and Chief Executive Officer
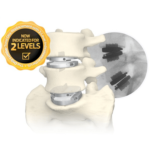
NGMEDICAL Announces Completion of Patient Enrollment in the one- and two-Level MOVE®-C Clinical Trial
NGMedical announces completion of patient enrollment for its multi-center one- and two-level trial, designed to support a future PMA-application for MOVE®-C. NONNWEILER, GERMANY, December 10, 2024 /EINPresswire.com/ -- NGMedical GmbH, a medical device manufacturer exclusively focused on creating inno-vative technologies for spinal application, announces completion … [Read more...] about NGMEDICAL Announces Completion of Patient Enrollment in the one- and two-Level MOVE®-C Clinical Trial
Countdown to Innovation: INNOVERSE Spinal System Launching December 16th!
We are excited to announce the official launch of the INNOVERSE Spinal System, set for December 16th! This state-of-the-art spinal fixation system is designed to revolutionize spinal surgery with its focus on optimized performance, flexibility, and diversity, ensuring exceptional outcomes for surgeons and patients alike. Optimized Performance The INNOVERSE Spinal System … [Read more...] about Countdown to Innovation: INNOVERSE Spinal System Launching December 16th!
Atlas Spine Announces FDA Clearance of its HiJAK Expandable Lateral Lumbar Interbody System
JUPITER, FL, December 10, 2024 — Atlas Spine, Inc., a spinal implant company based in Jupiter Florida, announced today they have received 510(k) clearance from the FDA for the HiJAK Expandable Lateral Lumbar Interbody System. Atlas is answering the evolving market needs of lateral fusion procedures with the introduction of HiJAK … [Read more...] about Atlas Spine Announces FDA Clearance of its HiJAK Expandable Lateral Lumbar Interbody System
Theradaptive Obtains FDA IDE Approval to Study OsteoAdapt™ SP in Additional Spinal Fusion Indications
FREDERICK, Md., Dec. 10, 2024 /PRNewswire/ -- Theradaptive, a privately held, clinical stage biologics company developing protein therapeutics for spine, orthopedics, soft tissue repair, and targeted immuno-oncology, announced today that the US Food and Drug Administration (FDA) has approved an expansion to its Phase I/II clinical program, broadening it to include … [Read more...] about Theradaptive Obtains FDA IDE Approval to Study OsteoAdapt™ SP in Additional Spinal Fusion Indications
PainTEQ Secures Groundbreaking Patent for LINQ® Technology Advancing Precision and Efficiency in SI Joint Fusion
TAMPA, Fla., Dec. 9, 2024 /PRNewswire/ -- PainTEQ, a leader in minimally invasive sacroiliac (SI) joint dysfunction treatment, has been awarded a new patent (US Patent No. 12,121,458), marking another significant milestone in the advancement of its innovative LinQ® SI Joint Stabilization System. This latest patent expands the breadth of PainTEQ's single-use instrumentation … [Read more...] about PainTEQ Secures Groundbreaking Patent for LINQ® Technology Advancing Precision and Efficiency in SI Joint Fusion
The Importance of Sales Representatives in the Operating Room: Supporting Spinal Surgeries
In the spine business, certain questions frequently arise: Is the presence of a sales representative in the operating room necessary? Does their involvement increase the company’s risk exposure? While these concerns are valid, the evidence points to a clear conclusion: sales representatives play a critical role in ensuring the success of surgical procedures. Their presence … [Read more...] about The Importance of Sales Representatives in the Operating Room: Supporting Spinal Surgeries
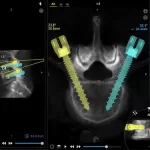
Study at HSS Compares Robotic-Assisted Navigation and Augmented Reality in Spine Surgery
A recent study conducted at the Hospital for Special Surgery (HSS) has shown that both robotic-assisted navigation (RAN) and augmented reality (AR) technologies achieve remarkable precision and safety in placing pedicle screws during spinal surgeries. The findings, published online ahead of print in the journal Spine, represent the first head-to-head comparison of these two … [Read more...] about Study at HSS Compares Robotic-Assisted Navigation and Augmented Reality in Spine Surgery
Curiteva Surpasses 2,000 Procedures With Inspire(R)C Cervical Trabecular PEEK(TM) With HAFUSE(R) Technology
December 4, 2024 (Newswire.com) - Curiteva, Inc., a privately held manufacturing and technology company, today announced it has implanted over 2,000 patients with the Inspire®C Cervical Interbody Fusion Device. This success translates to more than 3,700 implants placed, demonstrating the system's rapid adoption and effectiveness since its commercial launch in July … [Read more...] about Curiteva Surpasses 2,000 Procedures With Inspire(R)C Cervical Trabecular PEEK(TM) With HAFUSE(R) Technology
Tyber Medical Achieves Class III MDR CE Mark Certification from BSI for PEEK Titanium Plasma-Coated Cervical Cages
BETHLEHEM, Pa., Dec. 5, 2024 /PRNewswire/ -- Tyber Medical LLC, a leading provider of private-label orthopedic and spinal implants, proudly announces that it has received Medical Device Regulation (MDR) CE Mark certification from BSI for its PEEK Titanium Plasma-coated cervical cages. This certification for Tyber Medical's innovative technology is a pivotal step … [Read more...] about Tyber Medical Achieves Class III MDR CE Mark Certification from BSI for PEEK Titanium Plasma-Coated Cervical Cages
Introducing the FJ-f Interfacet Implant: Advancing Spinal Stabilization with Innovative Solutions
This year’s EUROSPINE 2024 symposium in Vienna is now behind us. It was a significant event for specialists in spine surgery. The gathering provided an excellent opportunity for showcasing innovations and exchanging experiences among surgeons from various countries. One of the program highlights was the presentation of the FJ-f intra-articular implant (Facet Joint … [Read more...] about Introducing the FJ-f Interfacet Implant: Advancing Spinal Stabilization with Innovative Solutions
NovApproach Spine Enters International Market
First Surgeries Using OneLIF™ Interbody Fusion Cage in Panama Alachua, Fla. Alachua, Fla. (December 4, 2024) – NovApproach Spine, developer of the patented OneLIF™ interbody spinal fusion system which supports a multitude of surgical approaches, announced today its entry into the Panamanian market with two surgeries performed last week. Dr. Gamaliel Gonzalez Atencio at the … [Read more...] about NovApproach Spine Enters International Market
Carlsmed Announces FDA Clearance for aprevo® Cervical Breakthrough Fusion Device
CARLSBAD, Calif.--(BUSINESS WIRE)--Carlsmed, Inc. (“Carlsmed” or the “Company”) a MedTech company pioneering AI-enabled personalized spine surgery, today announced FDA 510(k) clearance for the aprevo® Cervical ACDF Interbody System. This milestone underscores Carlsmed’s commitment to advancing patient-specific spine surgery solutions that enhance outcomes and surgical … [Read more...] about Carlsmed Announces FDA Clearance for aprevo® Cervical Breakthrough Fusion Device