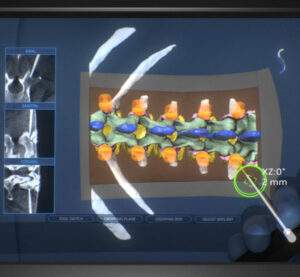
DEERFIELD, Ill., March 02, 2023 (GLOBE NEWSWIRE) — Surgalign Holdings, Inc. (Nasdaq: SRGA), a global medical technology company focused on elevating the standard of care by driving the evolution of digital surgery, today announced that Medical City Frisco, a world-class acute care hospital in Frisco, TX and part of HCA Healthcare, is the latest medical […]
2023
Aurora Spine Receives IRB Approval To Commence Multicenter Study for its DEXA-C™ Cervical Interbody System
CARLSBAD, Calif., March 02, 2023 (GLOBE NEWSWIRE) — Aurora Spine Corporation (“Aurora Spine” or the “Company”) (TSXV: ASG) (OTCQB: ASAPF), a designer and manufacturer of innovative medical devices that improve spinal surgery outcomes, today announced it has received Institutional Review Board (IRB) approval for its new multicenter study of its DEXA-C Cervical Interbody System, which […]
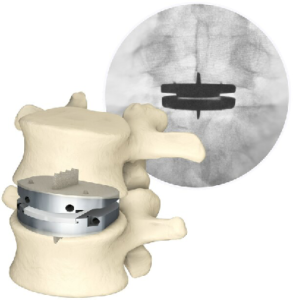
Aetna® Updates Disc Replacement Guidelines; All Major Payors Now Cover Centinel Spine’s Lumbar Total Disc Replacement
WEST CHESTER, Pa., March 2, 2023 /PRNewswire/ — Centinel Spine®, LLC, (“the Company”) a leading global medical device company addressing cervical and lumbar spinal disease through anterior surgical access, today announced significant expansion to patient access for the prodisc® L Lumbar Total Disc Replacement (TDR) system. An additional 22 million lives will now be covered as the result of […]
ZimVie Reports Fourth Quarter and Full Year 2022 Financial Results
WESTMINSTER, Colo., March 01, 2023 (GLOBE NEWSWIRE) — ZimVie Inc. (Nasdaq: ZIMV), a global life sciences leader in the dental and spine markets, today reported financial results for the fourth quarter and full year ended December 31, 2022. Management will host a corresponding conference call today, March 1, 2023, at 4:30 p.m. Eastern Time. “Our […]
Xtant Medical Acquires Coflex® Product Line from Surgalign for $17 Million
BELGRADE, Mont. and DEERFIELD, Ill., March 01, 2023 (GLOBE NEWSWIRE) — Xtant Medical Holdings, Inc. (NYSE American: XTNT), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, and Surgalign Holdings, Inc., (NASDAQ: SRGA), a global medical technology company focused on elevating the standard of care by driving the evolution of digital […]
Mediliant Group Announces New President of Their Facility in Plymouth, Indiana
LE LOCLE, SWITZERLAND (PRWEB) MARCH 01, 2023–In a continued effort to respond to customer needs and further boost company momentum, leaders in medical device contract manufacturing, Mediliant Group, has appointed industry expert Jason Ratcliffe as the new President of the organization’s CTE facility in Plymouth, Indiana. Mr. Ratcliffe has an extensive career in the manufacturing industry, […]
Induce Biologics Announces Launch of NMP Cancellous Strips
TAMPA, Florida—February 28, 2023—Induce Biologics USA, Inc., an orthobiologics company dedicated to the development of the next evolution of bone regeneration products, today announced the launch of its NMP Strip Bioimplant. The new product is an advanced cancellous bone-graft solution utilizing Induce Biologics’ proprietary Natural Matrix Protein (NMP) process, which unlocks the growth factors naturally found in bone, making them bioavailable. NMP […]
ATEC Reports Record Fourth Quarter and Full-Year 2022 Financial Results and Recent Corporate Highlights
CARLSBAD, Calif.–(BUSINESS WIRE)– Alphatec Holdings, Inc. (Nasdaq: ATEC), a provider of innovative solutions dedicated to revolutionizing the approach to spine surgery, today announced financial results for the quarter and full year ended December 31, 2022, and recent corporate highlights. Fourth Quarter and Full Year 2022 Financial Results Recent Highlights “I applaud the entire ATEC Family for delivering another record-breaking year,” […]
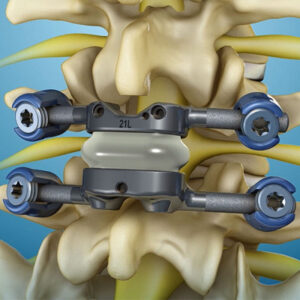
Premia Spine’s Tops™ Facet Joint Replacement Demonstrates Significant Improvement in all Patient-Reported Outcome Measures versus Fusion in FDA Trial
NORWALK, Conn. – Feb. 28, 2023 – Premia Spine, a medical technology company changing the way debilitating chronic leg and back pain is treated, today announced publication of two-year outcomes from the TOPS facet joint replacement system’s clinical trial in the Journal of Neurosurgery Spine. The study, “Prospective, Randomized and Controlled Multicenter Study of Posterior […]
(UPDATED 2023): +30 German Spinal Companies to Know…!
German thoraco-lumbar fixation market accounts fo 400 Million € with an annual growth around 3,5%. The key factors propelling the growth of this market are the increasing adoption rate of minimally invasive spinal surgeries, increasing technological advancements in spinal surgery, and increasing incidences of obesity and degenerative spinal conditions. The German spine market is led by companies […]
ZimVie Announces Launch of the RealGUIDE™ CAD and FULL SUITE Software Modules
WESTMINSTER, Colo., Feb. 23, 2023 (GLOBE NEWSWIRE) — ZimVie Inc. (Nasdaq: ZIMV), a global life sciences leader in the dental and spine markets, today announced the launch of the RealGUIDE™ CAD and FULL SUITE modules, the latest innovations within ZimVie’s digital dentistry software platform. The CAD (computer-aided design) module introduces advanced software for restorative design […]
Globus Medical Reports Fourth Quarter and Full Year 2022 Results
AUDUBON, Pa., Feb. 21, 2023 (GLOBE NEWSWIRE) — Globus Medical, Inc. (NYSE: GMED), a leading musculoskeletal solutions company, today announced its financial results for the fourth quarter and year ended December 31, 2022. Fourth Quarter 2022: Full Year 2022: “Globus Medical was founded twenty years ago with a small group of talented engineers who had a […]
Medtronic reports third quarter fiscal 2023 financial results
DUBLIN, Feb. 21, 2023 /PRNewswire/ — Medtronic plc (NYSE:MDT) today announced financial results for its third quarter of fiscal year 2023, which ended January 27, 2023. Key Highlights Medtronic reported third quarter worldwide revenue of $7.727 billion, a flat result as reported and an increase of 4.1% on an organic basis. The organic comparison excludes a $379 million negative impact from […]
Expanding Innovations, Inc. Announces FDA Approval of X-PAC Expandable Lateral Cage System
MOUNTAIN VIEW, Calif., Feb. 21, 2023 /PRNewswire/ — Expanding Innovations, Inc. (EI), a spinal implant company that designs NON-SCREW based expandable cage technology, announced today that the company has received 510(k) clearance from the FDA for the X-PAC Expandable Lateral Cage System (X-PAC LLIF). This approval marks a significant addition to the company’s expandable product portfolio which also includes the company’s flagship X-PAC Expandable […]
NuVasive Enhances C360 Portfolio with Expanded Indications for Modulus Cervical and Attrax Putty
SAN DIEGO, Feb. 21, 2023 /PRNewswire/ — NuVasive, Inc. (NASDAQ: NUVA), the leader in spine technology innovation, focused on transforming spine surgery with minimally disruptive, procedurally integrated solutions, today announced that it received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the use of its Modulus Cervical interbody implant with a bone void filler, further enhancing […]
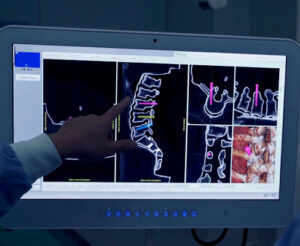
Software-Based VUZE Surgical Guidance System Demonstrates Benefits
RA’ANANA, Israel – February 21, 2023 – VUZE Medical, a privately-held company aiming to transform intra-operative guidance in minimally invasive spinal interventions, has successfully concluded a 20-patient clinical study for its VUZE System evaluating safety, accuracy and user experience at the Rambam Healthcare Campus, a 1,000-bed academic hospital in Haifa, Israel. The scope of the study was short-segment […]
Osseus Announces First Ever FDA Standalone Indication for Integrated ALIF with Alternative Fixation
DALLAS, TX, February 20, 2023 – Osseus, an innovative spinal solutions company, announces that the Pisces-SA has been granted the first ever FDA standalone indication for an integrated ALIF interbody utilizing alternative fixation (anchors) without the need for supplemental fixation. The primary goals of integrated fixation in ALIF interbodies are to prevent interbody expulsion and […]
Stryker’s Q Guidance System for cranial applications receives FDA clearance
Kalamazoo, Mich. – Stryker (NYSE:SYK), one of the world’s leading medical technology companies, today announced that its Q Guidance System with Cranial Guidance Software received 510(k) clearance from the U.S. Food and Drug Administration. The Q Guidance System is an image-based planning and intraoperative guidance system designed to support cranial surgeries. Recently launched in September 2022, the Q […]
Captiva Spine Expands Management Team to Support Roll-Out of the New Watchtower™ Spine Navigation and Robotics System
Captiva Spine is a medical device organization located in Jupiter, Florida, dedicated to delivering smart, elegant, and intuitive spinal fusion solutions. Today Captiva Spine announced the expansion of its management team to accelerate the roll-out of its WatchTower Spine Navigation and Robotic System. Daniel McPhillips has joined Captiva Spine as the Director of Enabling Technologies and Market […]
Why are Sales Reps a key piece in the success of the Globus and Nuvasive merger?
One of the reasons why acquisitions and mergers in the spinal market are complex is because of the great importance of the sales network in the success of those operations. Those of you who do not know this business will probably doubt it. But the reality is that the differentiation between the products of the […]
After some days…, What is our take on the merger Globus-Nuvasive?
As we said a few days ago, it is a brave move by Globus but necessary. As a spine-focused company with an ambition to lead the market, they had no choice but to acquire a company. We believe they have done the right thing. Globus could have bought a smaller one like Zimvie, Seaspine, or […]
About Globus Medical: History and Milestones!
The history of Globus is exciting if we take into account that in 20 years it has positioned itself as the leader in such a competitive and complicated market as Spine. Without a doubt, this success story is that of David Paul, a visionary and entrepreneurial genius.
ulrich medical USA® Announces First Percutaneous Spine Case for Momentum® Minimally Invasive Surgery System
ST. LOUIS, Feb. 14, 2023 /PRNewswire/ — ulrich medical USA, Inc., a privately held medical device technology company focused on developing and commercializing surgical solutions for treatment of spine pathologies in the United States, today announced the successful completion of the first case for the Momentum MIS System. The limited release of the MIS system represents new, increased functionality […]
Curiteva Inc. Announces FDA 510(k) Clearance for Inspire TM 3D Porous PEEK HA FUSETM Cervical Interbody System
Huntsville, AL based technology company, Curiteva announces the first FDA 510(k) cleared 3D printed PEEK implant, the Inspire Porous PEEK Cervical Interbody System with HAFUSE Technology. The Inspire platform is manufactured with a proprietary, patented Fused Filament Fabrication 3D printer designed, programmed, and built by Curiteva. This ground-breaking additive process produces a fully interconnected and integrated […]
Centinel Spine® Announces 500th Procedure with prodisc® C Vivo and prodisc C SK Cervical Total Disc Replacement System
WEST CHESTER, Pa., Feb. 14, 2023 /PRNewswire/ — Centinel Spine®, LLC, (“the Company”) a leading global medical device company addressing cervical and lumbar spinal disease through anterior surgical access, today announced the completion of the 500th procedure in the United States with its latest FDA-approved total disc replacement (TDR) system of prodisc® cervical solutions, prodisc C Vivo and prodisc C SK. The milestone […]
New Spineart Deformity System PERLA® TL LC – First Worldwide Surgeries
Spineart is proud to announce the first surgeries involving the PERLA® TL LC system, the new PERLA® TL add-on dedicated to the treatment of spine deformity. PERLA® TL LC is the extension of the PERLA® TL Posterior Thoracolumbar Fixation System and includes: Dr. DECKEY, Orthopaedic Specialty Institute (Orange, CA, USA) declared that “The PERLA® TL LC system is a clear advance for Spineart […]
We are proud to announce that LfC is Sponsor of SPINEMarketGroup in 2023!
Thank you LfC! On behalf of the SPINEMarketGroup team, we would like to thank you for supporting our site in 2023 through your PLATINUM sponsorship. About LfC LfC is a polish company who have achieved a leading position in the design and manufacture of surgical equipment used in spinal treatment in orthopaedics and neurosurgery.In the […]