CARLSBAD, Calif., July 12, 2022 (GLOBE NEWSWIRE) — Aurora Spine Corporation (“Aurora Spine” or the “Company”) (TSXV: ASG) (OTCQB: ASAPF), a manufacturer of innovative spinal implants, today announced the FDA clearance of a new Lumbar Spinal Stenosis Indication for Use for its ZIP™ family of MIS implants. Spinal Stenosis occurs when the spinal canal narrows […]
2022
IMPLANET Reports Its Revenue for Q2 2022, Up 33%
Bordeaux, Boston, July 11, 2022 – 06:00 pm CEST: IMPLANET (Euronext Growth: ALIMP, FR0013470168, eligible for PEA-PME equity savings plans), a medical technology company specializing in vertebral implants, today announced its revenue for the second quarter of 2022. Ludovic Lastennet, IMPLANET’s Chief Executive Officer, stated: “Despite the difficulties linked to the lack of personnel in […]
Safe Group announces half-year sales of €2.75 million, 27% growth
Eragny-sur-Oise, Fleurieux sur l’Arbresle, France, July 7th, 2022 5:45pm CET – Safe (FR0013467123 – ALSAF), a company specializing in the design, manufacturing and marketing of single-use technologies for spinal surgeries, delivering the safest treatment for spinal fractures urgently treated, announces its half-yearly revenues for the period ending June 30, 2022. “Safe Group closes the first half of 2022 […]
Surgalign™ Announces Clinical Site Expansion in the Commercialization of its HOLO Portal™ Surgical Guidance System in Ohio
DEERFIELD, Ill., July 06, 2022 (GLOBE NEWSWIRE) — Surgalign Holdings, Inc., (NASDAQ: SRGA) a global medical technology company focused on elevating the standard of care by driving the evolution of digital health, today announced continued momentum in the commercialization of its HOLO Portal surgical guidance system. The Company is pleased to report that Selvon St. […]
Additive Implants Announces Initial Surgeries for SureMAX-SA™, StandAlone Cervical Spacer
PHOENIX, July 05, 2022 (GLOBE NEWSWIRE) — Additive Implants, Inc., a medical technology company focused on addressing unmet spine surgeon needs, is pleased to announce the first implantations of the SureMAX-SA™ Cervical StandAlone, the third system in the family of SureMAX Cervical Spacers. The SureMAX-SA™ is a 3D-printed titanium device offering unmatched options in cervical spacer design. […]
Oxford Performance Materials Announces Agreement with Fuse Medical to Develop New Spinal and Sports Medicine Implants
SOUTH WINDSOR, CONN. (PRWEB) JULY 05, 2022 Oxford Performance Materials, Inc. (OPM), an industry leader in advanced polymer science and 3D printed orthopedic devices, announced today an agreement with Fuse Medical, Inc. (Fuse) a manufacturer and distributor of innovative medical devices, to develop new, world-class spinal, extremity and sports medicine implant product lines utilizing OPM’s patented OsteoFab® PEKK technology. […]
3Spine, Inc. Completes First US Surgeries
CHATTANOOGA, Tenn., June 29, 2022 /PRNewswire/ — 3Spine, Inc., a medical device company developing total joint replacement for the lumbar spine, today announced completion of the first series of US surgeries in the BalancedBack Total Joint Replacement Investigational Device Exemption (IDE) pivotal clinical trial. All patients enrolled in the IDE will receive the MOTUS device and will […]
NuVasive Opens Singapore Experience Center for Asia-Pacific region
SAN DIEGO, June 29, 2022 /PRNewswire/ — NuVasive, Inc. (NASDAQ: NUVA), the leader in spine technology innovation, focused on transforming spine surgery with minimally disruptive, procedurally integrated solutions, announced the grand opening of its Singapore Experience Center for the Asia-Pacific region, supporting the Company’s growth strategy to globalize its business. “The NuVasive Singapore Experience Center demonstrates our continued global commitment […]
Implanet Announces a Commercial, Technological and Financial Partnership With Sanyou Medical, a Chinese Manufacturer of Medical Devices for Spine Surgery
Bordeaux, Boston, June 29, 2022 – 05:45 pm CEST: IMPLANET (Euronext Growth: ALIMP, FR0013470168, eligible for PEA-PME equity savings plans), a medical technology company specializing in vertebral implants, announced today the signing of an agreement defining the principles of a strategic, technological and financial partnership project with Sanyou Medical, a Chinese manufacturer of medical devices […]
Medtronic receives spine industry’s first and only FDA clearance for breakthrough device indicated for ligament augmentation
Medtronic plc, a global leader in healthcare technology, has received U.S. Food and Drug Administration (FDA) 510(k) clearance and Breakthrough Device designation for its novel LigaPASS™ 2.0 Ligament Augmentation System. LigaPASS™ is the first and only FDA cleared device with indication for ligament augmentation in spine surgery. Ligament augmentation has been studied for its impact […]
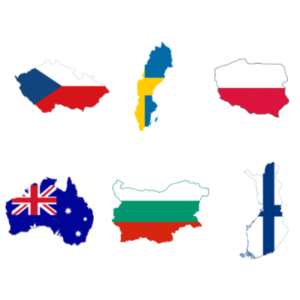
Learn about Spine Companies from the Czech Republic, Sweden, Poland, Australia, Bulgary, and Finland!
Today, we have expanded our spine companies section. We have incorporated companies from different places. Some, like Global biomedical, LfC, and others are highly recommended. We encourage you to take a look at them! The companies belong to the following six countries: Czech Republic Sweden Poland Australia Bulgary Finland We take this opportunity to remind […]
Cresco Spine closes seed investments to develop innovative dynamic surgical therapy for scoliosis
Cresco Spine, a new player in the spinal arena and a spin-off of the University Medical Center Utrecht (the Netherlands), today announced that it raised seed investments from InSpine and Utrecht Health Seed Fund (UHSF) to continue its development of their unique surgical spinal correction platform “Dynamic Implant SolutionsTM” (DISTM), initially focused on early onset […]
Spineway: New development dynamics and confirmed business strategy
Ecully, June 24th, 2022 – New momentum for a strong comeback on the French market A nice presence of Spineway at the SFCR Congress, the representative organization of the spinal surgical specialty in France, early June 2022 in Nice. This congress was the opportunity to present several new products for the first time and, in particular the ranges resulting from […]
3Spine, Inc. Announces IDE Approval
CHATTANOOGA, Tenn., June 22, 2022 /PRNewswire/ — 3Spine, Inc., a medical device company developing total joint replacement for the lumbar spine, today announced FDA Investigational Device Exemption (IDE) approval for their US pivotal clinical trial. The company previously completed contracting and site initiation at 16 US centers for a prospective real-world evidence (RWE) fusion study, which has […]
SeaSpine® Announces Full Commercial Launch of WaveForm® C 3D-printed Interbody System
CARLSBAD, Calif., June 21, 2022 (GLOBE NEWSWIRE) — SeaSpine Holdings Corporation (NASDAQ: SPNE), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today announced the full commercial launch of the WaveForm C Interbody System. The WaveForm C Interbody System, SeaSpine’s first cervical 3D-printed interbody, has the highest strength-to-porosity ratio compared […]
eCential Robotics and ChoiceSpine Announce Long-Term Partnership to Enhance Robotic Spine Implant Surgery
GIÈRES, GRENOBLE, France & KNOXVILLE, Tenn.–eCential Robotics, a French growth company that designs, manufactures, and markets the first unified 2D/3D robotic imaging and surgical navigation system for bone surgery indications and ChoiceSpine LLC, a US privately-held spinal implant company, announced today their long-term partnership to offer a common optimized solution. This system combines navigation, robotics, […]
Altus Spine announces FDA clearance of Sochi OCT system
Altus Spine has announced that it has received US Food and Drug Administration (FDA) 510(k) clearance of its Sochi OCT system—which is comprised of polyaxial screws, hooks, rods, locking screw assemblies and connectors which can be rigidly locked together in a variety of configurations to promote fusion for a wide variety of patient anatomies. “To […]
Bone healing with self-aware implants
(Sara Makaremi. Advancedsciencenews.com) –Smart implants have been developed with a wide range of functionalities. However, integrating the electronics for sensing, energy storage, and wireless communication into small devices creates significant challenges. “A viable solution to all of these challenges is to create a new class of smart implants that can utilize their constituent components to […]
Kleiner Device Labs to Present at McKinsey & Company Early Stage Investor Conference
June 16, 2022 — Incline Village, Nev., — Kleiner Device Labs (KDL) today announced that it will host a meeting room at McKinsey & Company’s Early Stage Investor Conference on the morning of Wednesday, June 22 as part of the event’s HealthTech track. Investors interested in talking with KDL may find more information and registration […]
Official Launch of the New Vertebral Fracture Reduction (VFR) System, TEKTONA®2
Spineart is proud to announce the launch of its upgraded, and now single-use, TEKTONA®2 Vertebral Fracture Reduction System, featuring enhanced ergonomics and usability. Aligned with the company’s goal of transforming spine surgery for patients, surgeons, and hospitals; Spineart has developed a minimally invasive technology that enables a targeted, controlled, and multidirectional reduction of the vertebral […]
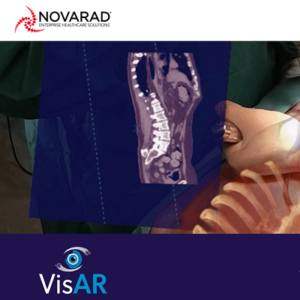
First Fully Immersive 3D Augmented Reality Surgical Navigation System Achieves FDA Approval for Precision Spine Surgery
SALT LAKE CITY, June 15, 2022 /PRNewswire/ — VisAR, an augmented reality surgical navigation system from healthcare technology leader Novarad™, receives FDA 510(k) approval for precision guided intraoperative spine surgery. VisAR transforms a patient’s imaging data into a 3-dimensional hologram which is visible through an optical visor and superimposed onto the patient with submillimeter accuracy. This allows the […]
What if innovation comes from leveraging the full potential of medical devices?
For years now, innovation efforts in spine surgery have been devoted to developing new treatments or finding new materials for implants. Little has been done to unleash the full potential of some of the most commonly used medical devices. Simple tools such as handle torques or surgical trays could however provide a wealth of information […]
Game-changing New Single-use Cervical and Mini-open Retractor to be Showcased at Becker’s Healthcare’s Spine, Orthopedic and Pain Management-Driven ASC Conference
SUNRISE, FL – On June 15, 2022, SURE Retractors Inc., a medical device company that has developed a range of industry-disruptive retractors for spinal surgery, announced it will be showcasing its new single- use Cervical and Mini-open Retractor at Becker’s Healthcare’s Spine, Orthopedic and Pain Management- Driven ASC Conference, which will take place June 16 […]
Xenco Medical Expands its Ambulatory Surgery Center Device Portfolio with FDA Clearance and Launch of its Multilevel CerviKit
SAN DIEGO–(BUSINESS WIRE)–Xenco Medical has expanded its ASC surgical device portfolio through the FDA clearance and launch of its Multilevel CerviKit™, an expansion of Xenco Medical’s breakthrough single-use cervical spine technology to include a comprehensive suite of implants and single-use instruments for 2, 3, and 4 level anterior cervical spine procedures. Optimized for the ambulatory […]
SI-BONE, Inc. Announces FDA Clearance for Expanded Indication of the iFuse-TORQ® Implant System
SANTA CLARA, Calif., June 13, 2022 (GLOBE NEWSWIRE) — SI-BONE, Inc. (SIBN), (Nasdaq: SIBN), a Silicon Valley-based medical device company dedicated to solving musculoskeletal disorders of the sacropelvic anatomy, today announced an FDA clearance for iFuse-TORQ® for pelvic fracture fixation, including acute, non-acute and non-traumatic fractures.* Fractures covered in this clearance include pelvic fragility fractures (fractures related […]
Accelus Announces Sales Leadership Appointments, Establishes U.S. East and West Sales Leaders
PALM BEACH GARDENS, Fla., June 13, 2022 (GLOBE NEWSWIRE) — Accelus, a privately held medical technology company focused on accelerating the adoption of minimally invasive surgery (MIS) as the standard of care in spine, today announced enhancements to its sales team with the promotion of Lisa Jacobs to Vice President of Sales, Jim Fox to […]
Aurora Spine Corporation Issued Patent by USPTO For Its Interlaminar Single Implant Technology For Motion Preservation or Fusion
CARLSBAD, Calif., June 13, 2022 (GLOBE NEWSWIRE) — Aurora Spine Corporation (“Aurora Spine” or the “Company”) (TSXV: ASG) (OTCQB: ASAPF), a designer and manufacturer of innovative medical devices that improve spinal surgery outcomes, today announced the issuance of United States Patent No: 11,331,199 entitled “Spinal Implant for Motion Preservation or Fusion.” This patent covers Aurora’s […]