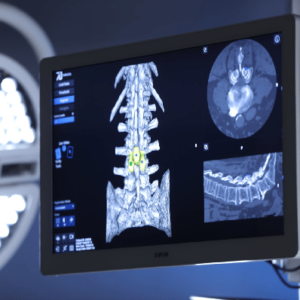
Todd H. Lanman, MD, and Jason M. Cuellar, MD, Ph.D., restore spinal motion in a woman with 4-level disc degeneration using “keelless” prodisc® C Vivo at three levels. LOS ANGELES, Oct. 18, 2022 (GLOBE NEWSWIRE) — ADR Spinal Restoration Center, Drs. Todd Lanman and Jason Cuellar are again breaking new ground in their pioneering efforts in multi-level […]
2022
SeaSpine Announces Full Commercial Launch of the 7D FLASH™ Navigation System Percutaneous Spine Module for Minimally Invasive Surgery
CARLSBAD, Calif., Oct. 18, 2022 (GLOBE NEWSWIRE) — SeaSpine Holdings Corporation (NASDAQ: SPNE), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today announced the full commercial launch of the 7D FLASH™ Navigation System Percutaneous Spine Module. The release of the Percutaneous Module represents a new application and increased […]
Accelus Announces Global Expansion of its FlareHawk Interbody Fusion System
PALM BEACH GARDENS, Fla., Oct. 18, 2022 (GLOBE NEWSWIRE) — Accelus, a privately held medical technology company focused on accelerating the adoption of minimally invasive surgery (MIS) as the standard of care in spine, today announced the rapid global expansion and international use of its FlareHawk® Interbody Fusion System. The FlareHawk System was recently cleared for […]
joimax® Experiences Major Growth, Launches TESSYS® TransSAP Endoscopic Surgical System
KARLSRUHE, Germany–(BUSINESS WIRE)–joimax® is continuing its year-over-year double digit growth. Over the past 9 months, joimax® has grown more than 25% globally and in the United States alone, more than 35%. Among this major spurt, the market leader in technologies and training methods for full-endoscopic and minimally invasive spinal surgery is launching a new endoscopic surgical system, […]
Welcome to Eurospine 2022! Who are the 113 Exhibitors?
EUROSPINE 2022 which will be held from October 19th to 21th in Milan, Italy. The congress will take place at the newly renovated MiCo – the Milan International Convention Centre. Below you can find the list of companies that exhibit there. To all of you who participate, we wish you a fantastic and productive Eurospine 2022! The […]
SPINEVISION will be part of EUROSPINE 2022 in Milan, Italy!
SPINEVISION will be part of EUROSPINE 2022 which will be held from October 19th to 21th in Milan, Italy. The company will present its Hexanium® technology, 3D printed titanium interbody cages, which brings numerous benefits to end-users, such as high porosity, strong stability and osseointegration capabilities. The SPINEVISION team will welcome you on booth n°66. About […]
NuVasive to Sponsor and Participate at EUROSPINE 2022
SAN DIEGO, Oct. 17, 2022 /PRNewswire/ — NuVasive, Inc. (NASDAQ: NUVA), the leader in spine technology innovation, focused on transforming spine surgery with minimally disruptive, procedurally integrated solutions, today announced it will continue its partnership with EUROSPINE as a gold sponsor and will attend EUROSPINE 2022 held October 19 to 21 in Milan. “Our support of EUROSPINE reflects our belief in […]
Official Launch of the New MIS Thoracolumbar Posterior Fixation System PERLA® TL MIS
Spineart is proud to announce the official full launch of its new Minimally Invasive Spine Surgery (MISS) System PERLA® TL MIS.Built on a successful limited release period, the new MIS system is ready for use worldwide. At Spineart, as an expert in Spine surgery, we strive to support all innovations that may support the transformation […]
What could be the consequences of the fusion of Orthofix and Seaspine?
This week we have published about the merger between Orthofix and Seaspine. This information has not surprised us since we knew several weeks ago that Seaspine had decided to leave the European market. It had already alerted us! It was unexpected to see Seaspine give up a relevant part of the world market. What happens […]
ulrich Medical USA™ Receives 510(k) Clearance for Flux-C™ 3D Printed Porous Titanium Cervical Interbody Prior to NASS
ST. LOUIS, Oct. 13, 2022 /PRNewswire/ — ulrich medical USA, Inc., a privately held medical device company focused on developing and commercializing musculoskeletal implant technologies in the United States, today announced the FDA has given 510(k) clearance of its Flux-C 3D printed porous titanium cervical interbody device. “Surgeons have many options for cervical interbodies. The Flux-C porous titanium device […]
Spineway:Significant increase of the turnover as of September 2022 compared with last year third quarter
Ecully, October 14, 2022 –Nine-month turnover of €5.4 million, up 82% “Over the past two years, our Group has undergone major changes and advances and has implemented its external growth plan. Indeed, the overall economic situation reinforces our belief that organic growth must be accompanied by external growth to return to a profitability objective. It is with this ambition that we acquired Distimp in mid-2021, enabling us to develop […]
Regulatory Clearance for the Align Interbody System with HAnano Surface
GOTHENBURG, SWEDEN AND DALLS (PRWEB) OCTOBER 13, 2022–Promimic AB, the world’s leading innovator in nano surface treatments for improved osseointegration, is pleased to announce a special 510(k) clearance from the U.S. Food and Drug Administration (FDA) for Acuity Surgical Devices, LLC. This is the second special 510(k) FDA clearance of a medical device with HAnano Surface, […]
Philips expands rollout of its ClarifEye augmented reality surgical navigation solution to Japan
October 13, 2022 International University of Health and Welfare, Mita Hospital, Tokyo, employs Philips’ augmented reality (AR) surgical navigation solution to treat first patients in Japan, helping to bring the patient benefits of minimally-invasive spine surgery to Japan’s aging population New data published in European Spine Journal on the clinical use of ClarifEye showed a […]
Stryker to Showcase Innovative Portfolio of Spine Solutions at the North American Spine Society Meeting
Kalamazoo, MICH., October 12, 2022 – Stryker (NYSE:SYK), one of the world’s leading medical technology companies, will feature its suite of procedural solutions for spinal surgery, including its award-winning Q Guidance System with Spine Guidance Software, during the North American Spine Society (NASS) annual meeting in Chicago, Oct. 12–15 (booth #4211). “Our expanding portfolio of […]
Bioventus to Showcase Full Orthobiologics and Ultrasonic Portfolio at NASS in Chicago
DURHAM, N.C., Oct. 12, 2022 (GLOBE NEWSWIRE) — Bioventus Inc. (Nasdaq: BVS) (“Bioventus” or the “Company”), a global leader in innovations for active healing, will present its spine procedural solutions portfolio at the North American Spine Society (NASS) meeting in Chicago, October 12 through 15. By combining the power of the neXus® BoneScalpel® for decompression with OSTEOAMP® […]
Welcome to NASS 2022! Who are the 300 Exhibitors?
NASS 2022 in Chicago has already started!In this edition, there are more than 300 exhibitors and companies. Below we include a link to the map of the organization and the complete list of companies that have a booth at the congress. Enjoy NASS! Complete Exhibitor List: 3DSYSTEMS 3Spine 4WEB Medical Accelus Accurate Neuromonitoring Acuity Surgical […]
ATEC Launches Expandable Technology for Medialized Posterior Procedure
CARLSBAD, Calif.–(BUSINESS WIRE)–Alphatec Holdings, Inc. (Nasdaq: ATEC), a provider of innovative solutions dedicated to revolutionizing the approach to spine surgery, announced that at the 2022 NASS Annual Meeting, the Company will introduce expandable interbody and access technology designed to enhance the medialized minimally-invasive (MIS) posterior approach. The procedure is designed to minimize muscle morbidity and blood […]
COMPaSS™ Personalized Spine Surgery Study Begins Enrollment
CARLSBAD, Calif.–(BUSINESS WIRE)–Carlsmed® announced today that the first patients have been enrolled in the COMPaSS™ study. COMPaSS, Clinical Outcome Measures in Personalized aprevo® Spine Surgery, is a multi-center post market prospective observational registry. The study will collect data on patients with degenerative spinal conditions and are treated surgically with Carlsmed aprevo devices and will track outcomes for a period of two years. “The […]
Nexus Spine Celebrates 5,000th Implantation of its Tranquil™ Interbody Fusion Technology
SALT LAKE CITY–(BUSINESS WIRE)–Nexus Spine, a developer of biomechanically-advanced solutions for spinal pathologies, today announced that its Tranquil™ interbody fusion technology has reached 5,000 implantations. The milestone spans across the company’s entire Tranquil™ portfolio consisting of cervical, cervical with integrated fixation, ALIF, ALIF with integrated fixation, PLIF, TLIF, Steerable TLIF, and DLIF configurations. Tranquil™ interbody devices are […]
It is NASS week and Paonan Biotech will be there!
Paonan Biotech, founded in 1985, dedicated in long-term spinal implant and external fixation system development as a professional medical device manufacturer. At NASS 2022, Paonan Biotech will bring brand-new services there and share many innovative projects and successful experience with you. Join Paonan Biotech in Chicago, October 13 to 15, at the NASS 2022 Annual […]
New Partners Wenzel Spine and Safe group join Forces during NASS, National American Spine Society, between October 11 and 14th 2022
Eragny-sur-Oise, France, Austin, Texas, USA, October 11th, 2022, 17h45 CET – Safe (FR0013467123 – ALSAF), a company specializing in the design, manufacturing, and marketing of ready-to-use technologies and services for spinal surgeries, and Wenzel Spine, a medical technology company focused on providing minimally invasive surgical and diagnostic solutions for the treatment of spinal disorders, having […]
Orthofix and SeaSpine to Combine in Merger of Equals to Create Leading Global Spine and Orthopedics Company
LEWISVILLE, Texas and CARLSBAD, Calif., Oct. 11, 2022 (GLOBE NEWSWIRE) — Orthofix (NASDAQ: OFIX), a global medical device company with a spine and orthopedics focus, and SeaSpine (NASDAQ: SPNE), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today announced they have entered into a definitive agreement to combine […]
CTL Amedica gains FDA clearance for MONDRIAN™ ALIF Cage System with Supplementary Fixation Plate
DALLAS (PRWEB) OCTOBER 11, 2022–CTL Amedica Corporation has received official 510k clearance from the U.S. Food and Drug Administration (FDA) to market its MONDRIAN™ Anterior Lumbar Interbody Fusion (ALIF) Cage System with Supplementary Fixation Plate. The MONDRIAN™ ALIF Cage System with Supplementary Fixation Plate, designated K213641/S003, is an integrated plate-and-cage construct, that can be manufactured in […]
Accelus Announces New Milestone with Its FlareHawk® Interbody Fusion System Surpassing 15,000 Devices Implanted
PALM BEACH GARDENS, Fla., Oct. 11, 2022 (GLOBE NEWSWIRE) — Accelus, a privately held medical technology company focused on accelerating the adoption of minimally invasive surgery (MIS) as the standard of care in spine, today announced that more than 15,000 FlareHawk® multiplanar expandable cages have been implanted in more than 11,000 patients in the United States. […]
Study Featured at NASS 2022 Demonstrates Long-Term Effectiveness of Centinel Spine’s prodisc® L Total Disc Replacement
WEST CHESTER, Pa., Oct. 11, 2022 /PRNewswire/ — Centinel Spine®, LLC, a leading global medical device company addressing cervical and lumbar spinal disease through anterior surgical access, today announced findings of a new study to be presented during the North American Spine Society (NASS) 37th Annual Meeting on Thursday, October 13, 2022. Thierry Marnay, MD will present results from the […]
ZimVie Expands Navigation-Compatible Platform to Include Posterior Cervical Application
WESTMINSTER, Colo., Oct. 11, 2022 (GLOBE NEWSWIRE) — ZimVie Inc. (Nasdaq: ZIMV), a global life sciences leader in the dental and spine markets, today announced the recent launch of the Virage Navigation System, an Occipital-Cervico-Thoracic (OCT) spinal fixation system featuring the innovative 360° Omnidirectional Extreme-Angle Screw. The Virage Navigation System simplifies rod alignment and minimizes […]
OsteoCentric Technologies Receives Two New 510k’s Prior to The North American Spine Society’s Annual Meeting
AUSTIN, Texas, Oct. 11, 2022 /PRNewswire/ — OsteoCentric Technologies, a privately held medical device company pioneering Mechanical Integration technology (UnifiMI™) for orthopedic and dental implants, has been issued two new FDA 510k clearances. The OsteoCentric Pedicle Screw Fastener System™ will feature UnifiMI technology, and the Integrity-SI Fusion System™ now includes indications in trauma surgery. Both systems will be featured at this month’s 37th Annual […]