Bordeaux & Boston, January 18, 2022 – IMPLANET (Euronext Growth: ALIMP, FR0013470168, eligible for PEA-PME equity savings plans), a medical technology company specialized in vertebral implants, today announced its 2021 fourth-quarter revenue, annul revenue and cash position at December 31, 2021. Ludovic Lastennet, IMPLANET’s CEO, said: “The strategic shift undertaken at the end of 2020 enabled […]
2022
Astura Medical Expands Sales Management Team
IRVING, TX – January 18, 2022 – Astura Medical, a high-growth, innovative spine technology company, announced today the addition of Tom Frazer as Area Vice President of Sales, Midwest, and Brett Heim as Area Vice President of Sales, Northeast. “We’re thrilled to welcome Tom and Brett to the Astura Medical Team,” said Steve Haayen, Vice […]
Genesys Spine is pleased to announce a record year for their SI Joint Division
AUSTIN, Texas, Jan. 18, 2022 /PRNewswire/ — Genesys Spine, a medical device manufacturer based in Austin, Texas, announced today that their SI Joint division exceeded 2021 sales targets: a 184% growth over 2020. “We are thrilled to reach this milestone and pleased to continue to contribute to pain relief. The Genesys Spine SI systems allows our physician partners the ability […]
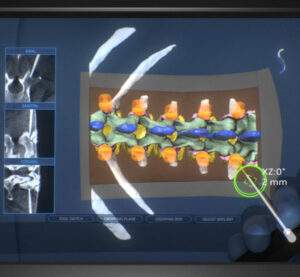
Surgalign Receives FDA Clearance for HOLO Portal™ System, the World’s First AI-driven AR Guidance System for Spine Surgery and Reports Preliminary Fourth Quarter and Full Year 2021 Results
DEERFIELD, Ill., Jan. 18, 2022 (GLOBE NEWSWIRE) — Surgalign Holdings, Inc., (NASDAQ: SRGA) a global medical technology company focused on elevating the standard of care by driving the evolution of digital health, today announced that it has received U.S. Food & Drug Administration (FDA) 510(k) clearance for its HOLO Portal surgical guidance system for use […]
VUZE Medical Announces U.S. FDA 510(K) Clearance for its VUZE System
RA’ANANA, Israel – January 18, 2022 – VUZE Medical, a privately held medical technology company aimed at transforming image guidance and verification in minimally invasive spine surgeries, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its VUZEÔ System. Using proprietary image processing, the VUZE System is a software-only solution that overlays a graphical […]
Amplify Surgical®, Inc. Hosts Inaugural Endoscopic Spine Symposium with Cadaver Lab – featuring dualX® and dualPortal™ System
IRVINE, CALIF. (PRWEB) JANUARY 17, 2022 Amplify Surgical, Inc., a medical device company focused on innovative minimally invasive surgery for the lumbar spine, will be holding its Inaugural Endoscopic Spine Symposium with Cadaver Workshop – featuring dualX® and dualPortal™ System, in Long Beach, California on February 5, 2022. Amplify Surgical is partnering with expert surgeons from […]
We are proud to announce that BIOMECH is Sponsor of SPINEMarketGroup again in 2022!
Thank you BIOMECH! On behalf of the SPINEMarketGroup team, we would like to thank you for supporting our site again in 2022 through your PLATINUM sponsorship. About BIOMECH Biomech is one the best orthopedic company in Taiwan for 27 years, focusing on designing/manufacturing spinal devices and instruments. Our goal is to decrease surgical process for […]
+20 French Spine Companies to Know…!
The French market for spinal fusion is a globally competitive market subject to rapid technological change, new product introductions and, other market activities of industry participants. The French market has been quite difficult for most foreign companies due to its low prices and the number of French competitors. We should not forget that French surgeons […]
VYRSA Technologies Launches SI Fusion Implant Offerings at 2022 North American Neuromodulation Society (NANS) Meeting
KING OF PRUSSIA, PA – January 13, 2022 – VYRSA Technologies, a specialty medical device firm located in King of Prussia, PA, has announced the release of a significant line-item extension to the company’s VYRSA™ Pro SI Fusion System portfolio. The VYRSA Pro SI Fusion System was one of the first posterior sacroiliac fusion systems […]
Inspan to showcase at NANS its newly released updated instruments for the percutaneous mini-open direct posterior technique for spinal fusion and stenosis treatment
MALDEN, MASS. (PRWEB) JANUARY 14, 2022 Inspan LLC, a Boston-based medical device company, is excited to announce at North American Neuromodulation Society (NANS) meeting on Jan 13-15, 2022 the release of its new Inspan “Mini” system down to the size of a standard laptop with the latest instruments and outpatient technique to perform lumbar spinal fusion […]
Safe group announces consolidated annual sales of €4.6M, up 23%
Eragny-sur-Oise, France, January 13th, 2022 17h45 CET – Safe (FR0013467123 – ALSAF), a company specializing in the design, manufacturing and marketing of single-use technologies for spinal surgeries, delivering the safest treatment for spinal fractures urgently treated, announces its 2021 annual revenue and its cash position. In 2021, the Group’s sales amounted to €4,636 thousand, up 23%, mainly due […]
We are proud to announce that PRODORTH is Sponsor of SPINEMarketGroup again in 2022!
Thank you PRODORTH! On behalf of the SPINEMarketGroup team, we would like to thank you for supporting our site again in 2022 through your PLATINUM sponsorship. Prodorth Spine RD Medical is dedicated to provide the best spinal implants & instruments to the market as it’s founded and supported by mechanical and materials engineers who are […]
First European Surgery Performed with the New JULIET®TI OL Cage Using the Insert Rotate Technique
Pr. Dr. El Hindy, St.Christphorus Krankenhaus GmbH (Werne, Germany) performed the first European JULIET® Ti OL I/R surgery during which he did a 4 levels fusion from L2 to S1 using the Insert/Rotate Technique for the 2 lower levels. Pr. Dr. El Hindy noted that – “To add sufficient lordosis the “Insert/Rotate cage” offers a clear advantage. It is intuitive […]
The Quantum Titanium Cervical Plate System showcases the benefits of additive manufacturing in bringing game-changing designs to market that ultimately impact patient care.
SAN ANTONIO – Nvision Biomedical Technologies and Watershed Idea Foundry have received FDA clearance for the first-ever completely additive manufactured titanium anterior cervical plate, the Quantum Titanium Cervical Plate System. Recently cleared, the Quantum system leads the way for game-changing design freedoms which push clinical benefits to new levels and ultimately impact patient care. The […]
We are proud to announce that Z-Medical GmbH is Sponsor of SPINEMarketGroup again in 2022!
Thank you Z-Medical GmbH! On behalf of the SPINEMarketGroup team, we would like to thank you for supporting our site again in 2022 through your PLATINUM sponsorship. About Z-Medical GmbH + Co. KG Based and founded in Tuttlingen in 2010, Z-Medical GmbH + Co. KG is a privately financed and held medical device company that […]
OrthoPediatrics Announces Preliminary Unaudited Revenue for the Fourth Quarter & Full-Year 2021
Jan 10, 2022–WARSAW, Ind., Jan. 10, 2022 (GLOBE NEWSWIRE) — OrthoPediatrics Corp. (“OrthoPediatrics” or the “Company”) (Nasdaq: KIDS), a company focused exclusively on advancing the field of pediatric orthopedics, today announced preliminary unaudited revenue for the fourth quarter and full year ended December 31, 2021. Preliminary unaudited fourth quarter 2021 revenue is expected to be $24.8 million, up 31%, when compared […]
ATEC Announces Preliminary 2021 Revenue Results and 2022 Guidance
CARLSBAD, Calif.–(BUSINESS WIRE)– Alphatec Holdings, Inc. (Nasdaq: ATEC), a provider of innovative solutions dedicated to revolutionizing the approach to spine surgery, announced today preliminary revenue results for the fourth quarter and full year ended December 31, 2021, and provided estimates for its full-year 2022 revenue guidance. Preliminary, Unaudited Q4 and Full-Year 2021 Revenue Ranges Preliminary, […]
SeaSpine Announces Preliminary Fourth Quarter and Full Year 2021 Financial Results, Including Record Fourth Quarter 2021 Revenue
CARLSBAD, Calif., Jan. 10, 2022 (GLOBE NEWSWIRE) — SeaSpine Holdings Corporation (NASDAQ: SPNE), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, announced today preliminary financial results for the fourth quarter and full year 2021. Fourth Quarter and Full Year 2021 Financial Results (all revenue amounts are preliminary and unaudited) […]
Inspan LLC November 24, 2021 Groundbreaking Achievement As the FIRST Interspinous Plate Fixation Device Granted FDA Clearance for Fusion and Spinal Stenosis from T1-S1
MALDEN, MASS. (PRWEB) JANUARY 10, 2022–Inspan has been FDA cleared since 2010 and thousands have been implanted safely with excellent clinical outcomes with no reported spinous process fractures or adverse device related events. A recent publication on Inspan in the spine journal being used to treat spinal stenosis at L4-5 with greater than five-year follow-up demonstrates […]
We are proud to announce that Viktor Hegedüs GmbH is Sponsor of SPINEMarketGroup again in 2022!
Thank you Viktor Hegedüs GmbH! On behalf of the SPINEMarketGroup team, we would like to thank you for supporting our site again in 2022 through your PLATINUM sponsorship. About Viktor Hegedüs GmbH The name Viktor Hegedüs has stood for extraordinary innovations and high-quality precision in the fields of medical technology and industrial applications for many years […]
NeuroStructures acquired by Incite Innovations
January 7, 2022 / – Incite Innovations has acquired Neurostructures. John Stephani (CEO) has confirmed it to SPINEMarketGroup this evening. Together with Moti Altarac, they leave a success story and a truly comprehensive and attractive product portfolio with the recent addition of expandable implants.The company falls into the best hands. Eric Hansen, CEO of Incite […]
We are proud to announce that Rudischhauser Surgical Instruments and Implants Manufacturing is Sponsor of SPINEMarketGroup again in 2022!
Thank you Rudischhauser Surgical Instruments and Implants Manufacturing! On behalf of the SPINEMarketGroup team, we would like to thank you for supporting our site again in 2022 through your PLATINUM sponsorship. About Rudischhauser Surgical Instruments and Implants Manufacturing Your partner and OE-Manufacturer of superior instruments, implants and complete set configurations for spinal, orthopaedic and trauma […]
WishBone and SpineGuard Sign National Distribution Agreement for US Pediatric Orthopedic Centers
WARSAW, Ind., Boulder, Co. and Paris, France – January 6, 2022 – 6:00 pm CET / 12:00 noon EST— WishBone Medical, Inc., a leader in pediatric orthopedic medical devices, and SpineGuard (FR0011464452 – ALSGD), an innovative company that deploys its Dynamic Surgical Guidance (DSG) sensing technology to secure and streamline the placement of bone implants […]
VySpine Announces FDA Clearance of the VySpan PCT System
Tallahassee, FL – January 6, 2021 – VySpine, a spine innovation leader using differentiated materials and designs, announced today that it has received 510(k) clearance from the FDA for the VySpan Posterior Cervical Thoracic System. The VySpan PCT System features various screw and hook options, multiple transition rods and revolutionary crosslink versatility. The variety of implant options […]
Stryker announces definitive agreement to acquire Vocera Communications
Kalamazoo, Michigan, Jan. 06, 2022 (GLOBE NEWSWIRE) — Stryker (NYSE: SYK) announced today a definitive merger agreement to acquire all of the issued and outstanding shares of common stock of Vocera Communications, Inc. (NYSE: VCRA) for $79.25 per share, or a total equity value of approximately $2.97 billion and a total enterprise value of approximately […]
Surgalign Holdings, Inc. Expands Digital Health Capabilities with Acquisition of a Significant Equity Interest in Inteneural Networks Inc.
DEERFIELD, Ill., Jan. 05, 2022 (GLOBE NEWSWIRE) — Surgalign Holdings, Inc. (NASDAQ: SRGA), a global medical technology company focused on elevating the standard of care through the evolution of digital health, today announced it has acquired an equity interest in Inteneural Networks Inc. (“INN”), and its proprietary artificial intelligence (AI) technology for autonomously segmenting and […]
NuVasive (NUVA) Granted FDA 510(k) Clearance for Expanded Indications of Attrax Putty
NuVasive, Inc. (NASDAQ: NUVA) announced the Company received U.S. Food and Drug Administration (FDA) 510(k) clearance for expanded indications of use for Attrax® Putty with its comprehensive thoracolumbar interbody portfolio for spine surgery. Attrax Putty is the first synthetic biologic to receive indications for use in interbody fusions of the thoracolumbar spine. The NuVasive Attrax Putty is a […]