STOCKHOLM, Feb. 8, 2022 /PRNewswire/ — Bactiguard and Zimmer Biomet have today agreed to expand the global license partnership, that was initiated in 2019, to cover multiple product segments. The extended exclusive license agreement covers implants for joint reconstruction (hips and knees), sports medicine, craniomaxillofacial and thoracic applications. It includes license and development fees of USD 1.5 million payable in […]
2022
ZimVie Hosts Inaugural Investor Day; Provides 2022 Financial Outlook
WARSAW, Ind. and WESTMINSTER, Colo., Feb. 7, 2022 /PRNewswire/ — ZimVie, the intended standalone, publicly traded entity to be spun off from Zimmer Biomet Holdings, Inc. (NYSE and SIX: ZBH), will host its inaugural Investor Day today, February 7, 2022, from 11:00 a.m. to approximately 2:00 p.m. Eastern Time. Investors can access the webcast here or by visiting Zimmer Biomet’s Investor Relations website at https://investor.zimmerbiomet.com. The virtual […]
Zimmer Biomet Announces Expected Completion Date of March 1, 2022 for Spinoff of ZimVie
WARSAW, Ind., Feb. 7, 2022 /PRNewswire/ — Zimmer Biomet Holdings, Inc. (NYSE and SIX: ZBH) today announced that its Board of Directors has declared a pro rata dividend of 80.3% of the outstanding common stock of ZimVie Inc. (“ZimVie”) to Zimmer Biomet shareholders of record as of the close of business on February 15, 2022 (the “Record Date”). The dividend […]
Aurora Spine Receives “Notice of Allowance” for Patent for Spinal Implant for Motion Preservation or Fusion
CARLSBAD, Calif., Feb. 07, 2022 (GLOBE NEWSWIRE) — Aurora Spine Corporation (“Aurora Spine” or the “Company”) (TSXV: ASG) (OTCQB: ASAPF), a designer and manufacturer of innovative medical devices that improve spinal surgery outcomes, announced today that it has received “Notice of Allowance” for its patent from the United States Patent and Trademark Office (USPTO), for […]
(UPDATED 2022) 125 Cervical Cages to Know…!
The global interbody fusion cage market is expected to reach $2,300 million by 2023.In recent years, cervical cages have evolved very much. The launch of stand alone and 3D technology cages has revolutionized this market. Today we have updated the list of these devices.The updated ACIF list has now 125 Stand Alone Cervical Cages. We […]
Kuros Biosciences’s MagnetOs Granules Cleared by FDA for Expanded Spinal Indications
SCHLIEREN (ZURICH), Switzerland, Feb. 03, 2022 (GLOBE NEWSWIRE) — Kuros Biosciences (“Kuros” or the “Company”), a leader in next generation bone graft technologies, announced today that its MagnetOs Granules has been cleared by the U.S. Food and Drug Administration (FDA) for expanded indications in the spine, making it only the second-ever bone graft to achieve […]
Empirical Spine Advances Limiflex™ as a New Motion-Preserving, Minimally Invasive Standard in Spine Surgery
SAN CARLOS, Calif., Feb. 1, 2022 /PRNewswire/ — Empirical Spine, Inc., a medical device company creating a new class of spinal implant that works in parallel with the natural structures of the spine to restore functionality and optimize quality of life, achieved several clinical, reimbursement and regulatory milestones in the past 12 months that are moving its LimiFlex™ Dynamic […]
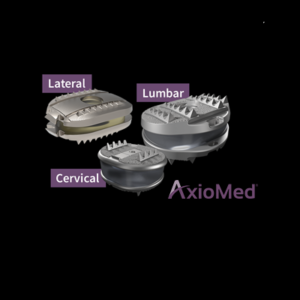
AxioMed LLC Files PMA Module II of III targeting 2022 FDA Approval of The First and Only Viscoelastic Lumbar Disc Replacement to Complete an IDE Clinical Study
MALDEN, MASS. (PRWEB) FEBRUARY 02, 2022–AxioMed’s lumbar viscoelastic total disc replacement PMA Module II was submitted to the FDA on January 28th, 2022. “The demand for disc replacement has been compounding over years in the USA, Europe and Australia and will J curve with the FDA approval of the superior AxioMed lumbar viscoelastic disc at the same […]
VySpine Announces FDA Clearance for ClariVy Cervical IBF System
Tallahassee, FL – February 1, 2021—VySpine, VySpine, a spine innovation leader using differentiated materials and designs, announced today that it has received 510(k) clearance from the FDA for the its ClariVy Cervical IBF System which is designed for use in anterior cervical discectomy with fusion (ACDF) procedures. The ClariVy Cervical IBF System features cervical interbody fusion devices made […]
2021 Spineway fiscal year results : significant improvement and sound dynamic
Spineway’s Board of Directors met on 31 January 2022 under the chairmanship of Stéphane Le Roux, and approved the annual accounts (corporate and consolidated) as of 31 December, 2021. Spineway confirms sound orientation of its 2021 fiscal year with a turnover of €4.3 million1 up 27% compared to 2020, despite the still penalizing pandemic context. This growth […]
NuVasive Appoints Andrew C. Morton as Chief Human Resources Officer
SAN DIEGO, Jan. 31, 2022 /PRNewswire/ — NuVasive, Inc. (NASDAQ: NUVA), the leader in spine technology innovation, focused on transforming spine surgery with minimally disruptive, procedurally integrated solutions, today announced the appointment of Andrew (Drew) C. Morton as senior vice president and chief human resources officer (CHRO) effective February 7, 2022. Mr. Morton brings more than 25 years of experience in human resources, […]
SpineGuard Files Its “510K” Dossier for the Clearance of Its “Threaded PediGuard” in Anterior Approach Spine Surgery
PARIS and BOULDER (CO), January 31, 2022 at 06:00 pm– SpineGuard (FR0011464452 – ALSGD), an innovative company that deploys its DSG® (Dynamic Surgical Guidance) sensing technology to secure and streamline the placement of bone implants, announced today the filing of its “510K” regulatory dossier with the FDA, seeking the authorization to commercialize its Threaded PediGuard device for […]
Stryker reports 2021 operating results and 2022 outlook
Kalamazoo, Michigan, Jan. 27, 2022 (GLOBE NEWSWIRE) — Stryker (NYSE:SYK) reported operating results for the fourth quarter and full year of 2021: Fourth Quarter Results Reported net sales increased 10.3% from 2020 and 13.8% from 2019 to $4.7 billion Organic net sales increased 9.0% from 2020 and 6.2% from 2019 Reported operating income margin of […]
SpineGuard reports 2021 revenue
PARIS and BOULDER (CO), January 26, 2022 at 06:00pm CET – SpineGuard (FR0011464452 – ALSGD), an innovative company that deploys its DSG® (Dynamic Surgical Guidance) sensing technology to secure and streamline the placement of bone implants, reported today its full-year 2021 revenue. Pierre Jérôme, Chairman, CEO and co-founder of SpineGuard, said: “In line with our prior quarters, […]
We are proud to announce that SpineVision is Sponsor of SPINEMarketGroup again in 2022!
Thank you SpineVision! On behalf of the SPINEMarketGroup team, we would like to thank you for supporting our site again in 2022 through your GOLD sponsorship. About SpineVision Since its creation SpineVision has played a pioneering role in the development and commercialization of innovative products for spinal surgery. The company offers unique, state-of-the-art, spinal systems […]
Biogennix Reports Record Year for Sales in 2021
IRVINE, Calif. — January 25, 2022 — Irvine-based Biogennix, an advanced osteobiologics company that develops, manufactures, and distributes proprietary bone grafting products used for bone fusion procedures, announced today that 2021 was the best sales year in company history. The company also announced that the third and fourth quarters of 2021 were the best performing six months […]
Bone Solutions Launches Mg OSTEOINJECT Injectable Magnesium-Based Bone Void Filler
COLLEYVILLE, Texas–Bone Solutions Inc. (BSI), an orthobiologics technology company located in Colleyville, Texas, announced today the immediate commercial launch of Mg OSTEOINJECT, the first injectable bone void filler in the U.S. to incorporate magnesium – a critical component for bone health and development. This flowable product is designed to promote bone repair and regeneration within insufficiency and […]
Aurora Spine Announces Initial Surgeries Using its Proprietary APOLLOTM Cervical Plate
CARLSBAD, Calif., Jan. 25, 2022 (GLOBE NEWSWIRE) — Aurora Spine Corporation (“Aurora Spine” or the “Company”) (TSXV: ASG) (OTCQB: ASAPF), a designer and manufacturer of innovative medical devices that improve spinal surgery outcomes, announced today that the first anterior cervical discectomy and fusion procedures were completed with its proprietary APOLLOTM Anterior Cervical Plate (ACP) system and […]
10 US Companies to Know…! (Part 1)
Today, in this first part, we highlight 10 companies. We consider that they have portfolios and products of great interest. There are more companies that we would like to include in this list. For this reason, next week we will publish the second part with additional 10 companies. US Spine Market The major factors attributing […]
Zimmer Biomet Announces Filing of Form 10 Registration Statement in Connection with Planned Spinoff of ZimVie; ZimVie to Host Investor Day on February 7
WARSAW, Ind. and WESTMINSTER, Colo., Jan. 21, 2022 /PRNewswire/ — Zimmer Biomet Holdings, Inc. (NYSE and SIX: ZBH) today announced that it has filed a Form 10 registration statement with the United States Securities and Exchange Commission (“SEC”) in connection with the intended spinoff of its Dental and Spine businesses into a standalone, publicly traded company, ZimVie. The Form […]
Spineart New Leadership Announcement
Effective January 1st, 2022, Jerome Trividic has been named Chief Executive Officer of the Spineart Group.Jerome joined Spineart in 2011 and has since worked side by side with the company’s founders as Group Chief Strategy Officer and President of Spineart US. Prior to joining Spineart, Jerome was an investment banker with Lazard specialized in the healthcare […]
NGMedical, Inc. Announces the Appointment of Mitch White as President and General Manager of its United States’ Business
SCOTTSDALE, Ariz., Jan. 20, 2022 (GLOBE NEWSWIRE) — NGMedical, a German medical device manufacturer exclusively focused on creating innovative technologies for spinal applications, announces the addition of Mitch White as President and General Manager. Mr. White has had a very distinguished career in the spinal implant industry with executive leadership roles for industry-leading companies including […]
We are proud to announce that Tsunami Medical is Sponsor of SPINEMarketGroup again in 2022!
Thank you Tsunami Medical! On behalf of the SPINEMarketGroup team, we would like to thank you for supporting our site again in 2022 through your PLATINUM sponsorship. About Tsunami Medical The company has been founded in 1997 as a subcontractor of some big manufacturing companies of invasive diagnostic devices. Over the years we bought the […]
Orthofix Announces Investment and Partnership with nView medical, Developer of Novel Imaging and Guidance Systems
LEWISVILLE, Texas–(BUSINESS WIRE)– Orthofix Medical Inc. (NASDAQ:OFIX), a global medical device company with a spine and orthopedics focus, and nView medical, a Salt Lake City-based company developing surgical imaging and guidance systems enabled by artificial intelligence (AI), today announced a partnership and investment agreement to jointly develop and co-market the innovative nView systems with Orthofix cervical […]
Amber Implants Closes US$10 M Series A Financing
The Hague, Netherlands, 19 January 2022: Amber Implants, an innovative medical technology company developing next generation spinal implants for spinal injuries, today announces the successful closing of its US$10 million Series A Financing in 2021. The round was co-led by the founders and existing venture capital investors, with participation from angel investors and non-dilutive government funding. […]
We are proud to announce that SIGNUS is Sponsor of SPINEMarketGroup again in 2022!
Thank you SIGNUS! On behalf of the SPINEMarketGroup team, we would like to thank you for supporting our site again in 2022 through your PLATINUM sponsorship. About SIGNUS SIGNUS – the Sign for Spine: Passionate! Dynamic! Worldwide! Innovative high-end implants made in Germany: For more than 25 years, SIGNUS has been the experienced specialist for […]
Spineway: Revenue increased by 27% in 2021 to €4.3 M Q4 2021 superior to Q4 2020 (+ 26%)
In a context still disrupted by the pandemic, Spineway closes the 2021 financial year with a turnover of €4.3 million, up 27% compared to 2020, driven by a strong commercial dynamic concretized by a 4th quarter at €1.3 million, up 26% compared to last year. This growth was driven in particular by high sales performances […]