MALDEN, MASS. (PRWEB) MARCH 22, 2022–Inspan is the first interspinous interlaminar spinous process fixation device to be FDA cleared for spinal stenosis and fusion. Harvard-Trained Orthopedic Spine Surgeon Dr. Erik Spayde sees a growing application for Inspan to be used in a single position lateral interbody cage with its ability to fixate the construct posteriorly. “I’ve […]
2022
How much and why is the Artificial Disc Market Growing?
The Artificial Disc market, according to DelveInsight is slated towards a positive market growth due to factors such as the increasing geriatric population, rising prevalence of degenerative disc disorders, a growing number of trauma cases and sports-related injuries, technological advancements and increasing product development activities. Furthermore, lower back pain is considered as one of the chief complaints pointing towards underlying spine-related disorder, increasing […]
SpineGuard’s first patent application on DSG-enabled robotic surgery allowed in the United States
PARIS and BOULDER (CO), March 21, 2022 at 06:00 pm CET – SpineGuard (FR0011464452 – ALSGD), an innovative company that deploys its DSG (Dynamic Surgical Guidance) sensing technology to secure and streamline the placement of bone implants, announced today that its first patent application directed to robotic DSG has been allowed by the United States […]
Life Spine Announces First Surgical Case of DYNA-LINK Titanium with Barb Fixation
Huntley, IL, March 14th, 2022 –Life Spine, a medical device company that designs, develops, manufactures and markets products for the surgical treatment of spinal disorders, announced today the first surgical cases of DYNA-LINK Titanium with Barb Fixation. DYNA-LINK Titanium Stand-Alone Anterior Lumbar System is a zero-profile, stand-alone device that offers surgeons a safe and reliable […]
Intech Reports Record Revenues in 2021 and Expansion Plans for 2022
MEMPHIS, TENN. (PRWEB) MARCH 16, 2022–Intech, the market leader in contract manufacturing of orthopedic medical devices, today reported 2021 financial results and provided a summary of recent business insights. Corporate Highlights: 2021 Group Revenue: Year-end revenues for 2021 reached $150M. These remarkable results are up 9% from the previous year’s total revenue. The growth was fueled […]
3Spine, Inc. Raises $33M in Private Series C Round
CHATTANOOGA, Tenn., March 15, 2022 /PRNewswire/ — 3Spine, Inc., a U.S. medical device company developing total joint replacement for the lumbar spine, has raised $33 million in an oversubscribed Series C private offering. Proceeds from the financing will be used for a Phase 2 clinical study of the BalancedBack Total Joint Replacement in the United States. To date the company has […]
Surgalign Holdings, Inc. Announces Fourth Quarter and Full Year 2021 Results
DEERFIELD, Ill., March 15, 2022 (GLOBE NEWSWIRE) — Surgalign Holdings, Inc., (NASDAQ: SRGA) a global medical technology company focused on elevating the standard of care by driving the evolution of digital surgery, today reported operating results for the fourth quarter and full year 2021. Recent Highlights: Total global spine revenue of $21.8 million, compared to […]
Safe Orthopaedics announces the signature of an agreement with the Clinicpartner buying group in Germany
Eragny-sur-Oise, France, March 10th, 2022 17h45 CET – Safe (FR0013467123 – ALSAF), a company specializing in the design, manufacturing and marketing of Ready-To-Use technologies for spinal surgeries, delivering the safest treatment for spinal fractures urgently treated, announces an exclusive agreement with the Clinicpartner buying group in Germany to supply their partner hospitals with it’s ready-to-use spinal implants and […]
What is the preliminary estimate of the spine market in 2022 including market shares?
The global spine market is estimated to grow 5% in 2022 to reach almost $10.500 billion.Today, we are going to make a preliminary market forecast for 2022. In some cases, we take the outlook 2022 published by some companies (Globus Medical or Nuvasive). In others, we are making our estimation. Please, take into account that […]
SeaSpine Announces Fourth Quarter and Full-Year 2021 Financial Results
CARLSBAD, Calif., March 11, 2022 (GLOBE NEWSWIRE) — SeaSpine Holdings Corporation (NASDAQ: SPNE), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, announced today financial results for the three-months and full-year ended December 31, 2021. Summary Fourth Quarter 2021 Financial Results and Recent Accomplishments Revenue of $55.6 million, an […]
Accelus Announces Successful First Surgeries Using Remi Robotic Navigation System with LineSider Spinal System
PALM BEACH GARDENS, Fla., March 10, 2022 (GLOBE NEWSWIRE) — Accelus, a privately held medical technology company focused on accelerating the adoption of minimally invasive surgery (MIS) as the standard of care in spine, today announced the successful completion of the first cases utilizing its Remi™ robotic targeting and navigation platform with its LineSider™ posterior […]
Southern Spine announces Center of Excellence partnership together with Integrated Pain Associates
March 1, 2022– Southern Spine, LLC, an ISO 13485:2016 certified manufacturer of implants and instruments for spinal surgery based in Macon, Georgia, announces that it has formed its first Center of Excellence together with Integrated Pain Associates (IPA) in Killeen, Texas. “We are excited to partner with IPA as we continue to train Interventional Pain […]
We are proud to announce that SpineGuard is Sponsor of SPINEMarketGroup again in 2022!
Thank you SpineGuard! On behalf of the SPINEMarketGroup team, we would like to thank you for supporting our site again in 2022 through your GOLD sponsorship. About SpineGuard Pierre Jérôme and Stéphane Bette founded SpineGuard in January 2009 with the goal of delivering the clinical benefits of the DSG™ (Dynamic Surgical Guidance) technology to as […]
CoreLink® Announces First of its Kind Fusation™ 3D Printed Cervical Anchor
ST. LOUIS, March 8, 2022 /PRNewswire/ — CoreLink, LLC, a leading designer and manufacturer of spinal implant systems, today announced 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the addition of Fusation™ anchors to the F3D-C2 Cervical Stand-alone Fusion System. The Fusation anchors are an ideal alternative to traditional fixation screws for surgical scenarios when […]
Xtant Medical Announces Fourth Quarter and Full Year 2021 Financial Results
BELGRADE, Mont., March 08, 2022 (GLOBE NEWSWIRE) — Xtant Medical Holdings, Inc. (NYSE American: XTNT), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today reported financial and operating results for the fourth quarter and year ended December 31, 2021. “Xtant accomplished numerous objectives in 2021 focused on developing […]
VySpine Announces FDA Clearance for LumiVy Lumbar IBF System
Tallahassee, FL – March 8, 2022—VySpine, a spine innovation leader using differentiated materials and designs, announced today that it has received 510(k) clearance from the FDA for its LumiVy Lumbar IBF System which is designed for use after lumbar discectomy in fusion procedures. The LumiVy Lumbar IBF System features lumbar interbody fusion devices made from either […]
SeaSpine® Announces New Senior Leadership Team Appointments
CARLSBAD, Calif., March 07, 2022 (GLOBE NEWSWIRE) — SeaSpine Holdings Corporation (NASDAQ: SPNE), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today announced the elevation of Dr. Frank Vizesi to Chief Scientific Officer and Shaeffer Bannigan to Vice President, Product Development, Spinal Implants. With his promotion, Mr. Bannigan joins […]
We are proud to announce that A-SPINE is Sponsor of SPINEMarketGroup in 2022!
Thank you A-SPINE! On behalf of the SPINEMarketGroup team, we would like to thank you for supporting our site in 2022 through your PLATINUM sponsorship. About A-SPINE The leading spine medical device brand in Taiwan A-SPINE Asia Co., Ltd. (A-SPINE) was founded in 2001. It is the first company in Taiwan to focus on the R&D, […]
More US Companies to Know…! (Part 2)
Today, in this second part, we want you to know about another 10 companies. We consider that they have portfolios and products of great interest. There are many more US companies that we would like to include in this list. For this reason, we will publish soon the third part with additional companies. We have […]
Aurora Spine Corporation Announces Initial Implantation of the World’s First Bone Density Matched DEXA-C Cervical Interbody Fusion Device
CARLSBAD, Calif., March 03, 2022 (GLOBE NEWSWIRE) — Aurora Spine Corporation (“Aurora Spine” or the “Company”) (TSXV: ASG) (OTCQB: ASAPF), a manufacturer of innovative spinal implants announced today that it has completed the world’s first DEXA-C implantation at Cypress Pointe Surgical Hospital in Louisiana, where the implant density was directly matched with the patient’s bone […]
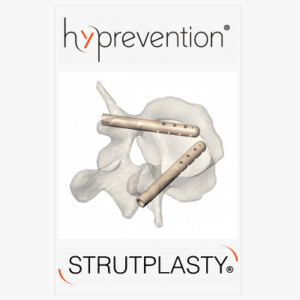
Hyprevention raises $4.5M to accelerate commercialization of the V-STRUT© Vertebral Implant product
NEW YORK, NY, USA, March 2, 2022 /EINPresswire.com/ — Hyprevention has developed the STRUTPLASTY® Platform intended to reinforce bone in case of fractures or impending fractures. The technology is protected by 25 patents, in the United States and Europe. Two products are currently on the market and the platform will be expanded to address more anatomical sites. In 2020, V-STRUT© […]
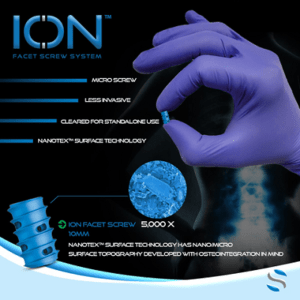
SurGenTec Launches ION Screw, Smallest Posterior Spinal Fixation Implant, to Market After Receiving FDA Clearance
BOCA RATON, Fla.–(BUSINESS WIRE)–SurGenTec®, a privately held spine and orthopedic technology company based in Boca Raton, FL, announced that it has received clearance from the U.S. Food and Drug Administration (FDA) for ION™ Screw, its proprietary stand-alone spine fixation implant. This unique device can be used to treat a variety of pathologies throughout the spine […]
SeaSpine® Announces Full Commercial Launch of NorthStar™ Facet Fusion and Limited Commercial Launch of FLASH™ Navigation Lumbar Facet Fusion
CARLSBAD, Calif., March 01, 2022 (GLOBE NEWSWIRE) — SeaSpine Holdings Corporation (NASDAQ: SPNE), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today announced the full commercial launch of NorthStar™ Cervical Facet Fusion and the limited commercial launch of FLASH™ Navigation Lumbar Facet Fusion, the latest orthobiologics solutions tailored […]
We are proud to announce that Normmed is Sponsor of SPINEMarketGroup in 2022!
Thank you Normmed! On behalf of the SPINEMarketGroup team, we would like to thank you for supporting our site in 2022 through your PLATINUM sponsorship. About Normmed Normmed makes a difference in both domestic and international markets with innovative solutions in its field and progresses on this path. Normmed, which manufactures spinal implants and instruments […]
SMADE is Proud to Announce its Official Launch and its Ambition to Be Instrumental in the Future of Smart Healthcare
After a 5 year incubation period within Intech, the WAYVIO project gave birth to SMADE in January 2022, a stand-alone IoMT company providing data-driven smart solutions for OEMs and hospitals. With offices in Chicago and Lyon, SMADE is on track to serve both the U.S and the European markets with its cutting-edge technology. IT ALL […]
ATEC Reports Fourth Quarter and Full Year 2021 Financial Results and Recent Corporate Highlights
CARLSBAD, Calif.–(BUSINESS WIRE)– Alphatec Holdings, Inc. (Nasdaq: ATEC), a provider of innovative solutions dedicated to revolutionizing the approach to spine surgery, today announced financial results for the quarter and full year ended December 31, 2021, and recent corporate highlights. Recent Highlights Accelerated adoption of Prone Trans-Psoas (PTP) Technique, with ATEC lateral procedures delivering over 40% of […]
SpineUp Announces U.S. FDA 510(K) Clearance for its New Cervical Cage in Peek HA-Enhanced
February 28, 2022– SpineUp a privately held medical technology company has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its brand new cervical cage in Peek Optima HA-Enhanced, Romero.They offer to the surgeons 4 types of cervical cages: Lordotic with screw (self-tapping or self-drilling), anatomic with screw, anatomic and lordotic without […]