PALM BEACH GARDENS, Fla. and SUMMIT, N.J., April 15, 2022 (GLOBE NEWSWIRE) — Integrity Implants Inc. d/b/a Accelus (“Accelus”), a privately held medical technology company focused on accelerating the adoption of minimally invasive surgery (MIS) as the standard of care in spine, and CHP Merger Corp. (“CHP”) (Nasdaq: CHPM, CHPMU and CHPMW), a special purpose […]
2022
Spineway: 2022 first quarter : Income increase by 55%
In line with the last quarter of 2021, Spineway confirms its growth momentum and totals revenues of €1.3 million for the first quarter of 2022, up 55% compared to the year 2021. The latter benefits from the synergies implemented with Distimp, the growth of sales in France and positive commercial orientation in Latin America. Sales in Latin America showed strong growth […]
First Surgery Worldwide with IDYS®-C ZP 3DTi!
The first surgery with the new Idys-C ZP 3DTi was successfully performed by Dr. Patrick Guérin in France (Centre Aquitain du dos – Bordeaux).Idys-C ZP 3DTi is Clariance’s new ZERO PROFILE CERVICAL CAGE. Benefits: ANATOMICAL SHAPEThe anatomical shape of the cage conforms to the patient’s cervical vertebrae for enhanced stability. ZERO PROFILEZero anterior profile to […]
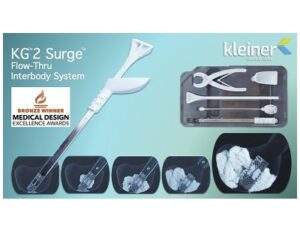
Kleiner Device Labs’ KG2 SurgeFlow-Thru InterbodySystem
April 13, 2022 — Incline Village, Nev., — Kleiner Device Labs today announced that its new KG2TM SurgeTM flow-thru interbody system was recognized with the Bronze award in the 2022 Medical Design ExcellenceAwards (MDEA) in the Implant and Tissue-Replacement Products category. The KG2 system maximizes total bone graft delivery volume, better distributes graft bilaterally into […]
OrthoPediatrics Spotlights Award-Winning ApiFix® Technology at IMAST
WARSAW, Ind., April 12, 2022 (GLOBE NEWSWIRE) — OrthoPediatrics Corp. (“OrthoPediatrics” or the “Company”) (Nasdaq: KIDS), a company exclusively focused on advancing the field of pediatric orthopedics, showcased its award-winning technology, the ApiFix® System, at the 2022 International Meeting on Advanced Spine Techniques (IMAST) held in Miami, Florida April 6-9, 2022. The IMAST meeting offered a comprehensive program […]
Fuse Medical, Inc. Announces Appointment of New CFO Lawrence S. Yellin
RICHARDSON, Texas–(BUSINESS WIRE)–Fuse Medical, Inc. (OTCPINK: FZMD) (“Fuse” or the “Company”) an emerging manufacturer and distributor of innovative medical devices for the orthopedic and spine marketplace, announced the appointment of new Chief Financial Officer and member of the Executive Board, Lawrence (“Larry”) S. Yellin. As CFO, Mr. Yellin will be responsible for all of the […]
SeaSpine Announces Full Commercial Launch of the Reef® TA (TLIF Articulating) Interbody System
CARLSBAD, Calif., April 11, 2022 (GLOBE NEWSWIRE) — SeaSpine Holdings Corporation (NASDAQ: SPNE), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today announced the full commercial launch of the Reef TA (TLIF Articulating) Interbody System. The Reef TA Interbody System is designed to reliably deliver an interbody to […]
restor3d Raises $23 Million to Expand 3D Printed Personalized Solutions for Musculoskeletal Surgery and Develop Machine Learning Enabled Design Tools
DURHAM, N.C.–restor3d, a leading medical device company and 3D printing manufacturer, has raised $23 million to expand the delivery of 3D printed personalized surgical solutions across multiple musculoskeletal specialties and to develop machine learning software tools for assisting engineers with patient specific implant design. Ken Gall Ph.D., co-founder of restor3d and a Professor at Duke […]
Major Commercial Third Party Payer in Utah and Idaho Significantly Expands Patient Access to Centinel Spine’s prodisc® L for One- and Two-level Lumbar Total Disc Replacement
WEST CHESTER, Pa., April 5, 2022 /PRNewswire/ — Centinel Spine®, LLC, a leading global medical device company addressing cervical and lumbar spinal disease through anterior surgical access, today announced significant expansion of coverage for lumbar total disc replacement (TDR) procedures in Utah and Idaho. This policy expansion announced by a major commercial third party payer represents 837,000 covered lives across […]
Astura Medical Receives FDA 510(k) Clearance For El Capitan Oblique Anterior Lumbar Interbody Fusion System
IRVING, TX – April 4, 2022 – Astura Medical, a high-growth, innovative spine technology company, today announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its El Capitan Oblique Anterior Lumbar Interbody Fusion (ALIF) System. Built from the foundation of success with the company’s other integrated plate and […]
SpineGuard obtains FDA clearance for commercial release of its “Threaded PediGuard” in anterior approach spine surgery
PARIS and BOULDER (CO), April 4, 2022, at 06:00pm CEST – SpineGuard (FR0011464452 – ALSGD) (Paris:ALSGD), an innovative company that deploys its DSG (Dynamic Surgical Guidance) sensing technology to secure and streamline the placement of bone implants, announced today the authorization under 510K #220160 by the FDA (Food and Drug Administration) to commercialize in the United […]
Amplify Surgical®, Inc. Announces the First Surgical Case using 15-Degree Lordotic dualX® Dual Expanding Interbody Fusion Implant
IRVINE, CALIF. (PRWEB) APRIL 04, 2022–Amplify Surgical, a medical device company focused on dual expanding interbody technologies, announced the launch of the dualX T/PLIF 15-degree lordotic interbody cages. Dr. Stefan Renaud became the first spine surgeon to implant the new 15-degree lordotic dualX interbody cage, a TLIF spacer that provides additional lordosis. The minimally invasive procedure […]
ZimVie Reports 2021 Financial Results, Reaffirms 2022 Financial Guidance, and Provides Supplemental Financial Information
WESTMINSTER, Colo., March 31, 2022 (GLOBE NEWSWIRE) — ZimVie Inc. (Nasdaq: ZIMV), a global life sciences leader in the dental and spine markets, today reported financial results for the three months and full year ended December 31, 2021. “We are intently focused on strengthening our foundation in the $20 billion global dental and spine markets […]
CTL Amedica Corp Increases International Footprint: Receives Two Product Registration Certifications from Guatemala Ministry of Public Health and Social Assistance
DALLAS (PRWEB) MARCH 31, 2022–The Guatemala Ministry of Public Health and Social Assistance has certified CTL Amedica Corporation’s registration for two product lines: the VALEO™ II TL Silicon Nitride Interbody Fusion Devices, and the RAPHAEL™ Pedicle Fixation Platform. This certification will enable CTL Amedica to market these products in Guatemala. This announcement marks CTL Amedica’s first product registration […]
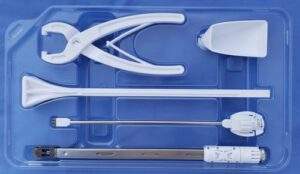
Kleiner Device Labs’ KG2 Surge Flow-Thru Interbody System Named Finalist for MD+DI Medical Design Excellence Award
INCLINE VILLAGE, Nev., March 31, 2022 /PRNewswire/ — Kleiner Device Labs today announced that its new KG2TMSurgeTM flow-thru interbody system has been selected as a finalist in the 2022 Medical Design Excellence awards in the Implant and Tissue-Replacement Products category. The KG2 system maximizes total bone graft delivery volume, better distributes graft bilaterally into the intervertebral disc space, […]
We are proud to announce that BAUI is Sponsor of SPINEMarketGroup in 2022!
Thank you BAUI! On behalf of the SPINEMarketGroup team, we would like to thank you for supporting our site in 2022 through your PLATINUM sponsorship. About BAUI BAUI is one of the leading medical devices companies in Taiwan. Since 2009, BAUI has started the spinal devices supply with its own brand. The main products include […]
Life Spine Announces Additional 510k Clearance for SImpact Si Joint Fixation System
Huntley, IL, March 29th, 2022 –Life Spine, a medical device company that designs, develops, manufactures and markets products for the surgical treatment of spinal disorders, announced today that is has received clearance from the U.S. Food & Drug Administration (FDA) to market SImpact Si Joint Fixation System for Posterior-Oblique approaches. Whether the physician is performing […]
Spine Innovations Marks Milestone — Over 20,000 ESP Spinal Discs Implanted — as Global Demand Grows
HEIMSBRUNN, France–Since its formation as a wholly independent company in late 2020, Spine Innovations has continued to expand sales of its ESP spinal disc replacements, with more than 20,000 prostheses successfully implanted in patients around the world. At the same time, Spine Innovations continues to expand its market reach. The company is now active in […]
HAPPE Spine Announces News Additions to Board of Directors
PRNewswire/ — HAPPE Spine, an emerging leader in the development of next generation orthopedic and spinal implants enabled by the HAPPE™ material platform, announces strategic new additions to its Board of Directors. Following a $3.35 million Series A capital raise, a new five-member Board of Directors was established to strengthen leadership as HAPPE Spine moves towards its first FDA […]
Historical Spine Systems (1) : Dick´s “Fixateur Interne”
The transpedicular fixation was initially developed by Dr Magerl in 1977. Originally it was used for external fixation of the lower thoracic and lumbar spine. The fixation device, consisting of two transverse bars and three threaded rods with triangular locking plates, provided rigid stability. The system could be applied in distraction, compression, or in a […]
Globus Medical, Expected to Post Quarterly Sales of $235.53 Million
Equities analysts expect Globus Medical, Inc. (NYSE:GMED) to report sales of $235.53 million for the current quarter, according to Zacks Investment Research. Five analysts have made estimates for Globus Medical’s earnings. The highest sales estimate is $243.24 million and the lowest is $230.81 million. Globus Medical posted sales of $227.34 million in the same quarter last […]
Xlife Sciences and curasan form joint venture company to research biosurgical treatment therapies
Xlife Sciences AG (SIX: XLS) and curasan AG, a leading global provider of biomaterials for bone and tissue regeneration in dental and orthopedic surgery, have formed the joint biosurgical company Novaxomx. The joint venture, Novaxomx GmbH, is based at the Eisenberg campus of the University Hospital Jena (Germany) and focuses on the research, development, certification, […]
Safe Group ships several dozen ready-to-use surgical kits to two hospitals in Ukraine
Eragny-sur-Oise, France, March 25th, 2022 08h45 CET – Safe (FR0013467123 – ALSAF), a company specializing in the design, manufacturing and marketing of single-use technologies for spinal surgeries, delivering the safest treatment for spinal fractures urgently treated, announces today the shipment of several dozen complete ready-to-use surgical kits (implants and instruments) to two hospitals: Dnipro Military Hospital (neurosurgery department, […]
SeaSpine® Announces 50,000th NanoMetalene® Implantation
CARLSBAD, Calif., March 24, 2022 (GLOBE NEWSWIRE) — SeaSpine Holdings Corporation (NASDAQ: SPNE), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, announced today the implantation of the 50,000th NanoMetalene® interbody device. NanoMetalene is a proprietary surface technology for interbody implants that incorporates a sub-micron layer of commercially pure titanium […]
New EVoluSIon Study Data Demonstrates Strong Support for the SImmetry® Sacroiliac Joint Fusion System
DEERFIELD, Ill., March 24, 2022 (GLOBE NEWSWIRE) — Surgalign Holdings, Inc. (NASDAQ: SRGA), a global medical technology company focused on elevating the standard of care by driving the evolution of digital health, today announced the publication of results from EVoluSIon (EVSI), a large, prospective, multi-center study of clinical outcomes following minimally invasive sacroiliac joint (SIJ) […]
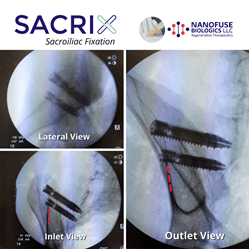
Sacrix The First To Use Navigation To Perform A Percutaneous Lateral-Oblique Sacroiliac Joint Fixation
MALDEN, MASS. (PRWEB) MARCH 22, 2022–Navigation in spine surgery was invented as a powerful technology to aid in placing pedicle screws with increased safety, efficiency, and reproducibility. Sacrix saw its chance to expand the use of navigation in placing its sacroiliac joint screws. Sacrix therefore jumped at the opportunity when a neurosurgeon felt that he would […]
Introducing Velocity Medtech: An Integrated Solutions Provider Revolutionizing Orthopaedic Medical Manufacturing
TOTOWA. N.J., March 22, 2022 /PRNewswire/ — Velocity Medtech (Velocity), announced today the official formation of the organization that unifies brands with the mission to simplify manufacturing and manufacturing services as an integrated partner to leading orthopaedic implant and instrument medical device organizations around the world. Velocity brings together the proven medical manufacturing expertise of Medin Technologies […]