DALLAS (PRWEB) MAY 18, 2022–CTL Amedica Corporation has received official 510k clearance from the U.S. Food and Drug Administration (FDA) to market the CTL Amedica Navigation Instrument System, registration number K213491. The system features manual surgical instruments adaptable for use with third-party navigation systems, which are designed to assist surgeons in precisely locating anatomical structures in […]
2022
Biogennix’s DirectCell Advanced Bone Grafting System Used in 500th Case
IRVINE, Calif. — May 17, 2022 — Irvine-based Biogennix, a market leader in advanced bone regeneration technology, announced today that its DirectCell Advanced Bone Grafting System has already been used in over 500 cases. Biogennix’s team of bone regeneration experts specifically designed this system to provide surgeons and hospitals with a comprehensive bone graft solution in a single product. The DirectCell […]
100 U.S. Patients Treated in the single-level IDE Clinical Trial of BAGUERA® C Cervical Disc Prosthesis
LAGUNA HILLS, CA, April 21, 2022 – Spineart USA Inc. announced today that surgery has been performed on 100 patients in the single-level U.S. Investigational Device Exemption (IDE) Clinical Trial of the BAGUERA® C Cervical Disc Prosthesis. The BAGUERA® C implant is an investigational device in the U.S. designed to reconstruct the cervical disc following […]
Amplify Surgical®, Inc. Announces First Official dualPortal™ Endoscopic Technique Surgeon Training in Michigan
IRVINE, CALIF. (PRWEB) MAY 12, 2022–Amplify Surgical, Inc., a medical device company focused on innovative minimally invasive surgery for the lumbar spine, is pleased to announce the successful completion of the first official dualPortal Endoscopic Technique Training since Amplify Surgical’s Inaugural Endoscopic Spine Symposium. The training was facilitated by Dr. Daniel K. Park, board certified orthopedic […]
Accelus Receives U.S. FDA 510(k) Clearance for its FlareHawk TiHawk11 Interbody Fusion System
PALM BEACH GARDENS, Fla., May 12, 2022 (GLOBE NEWSWIRE) — Accelus, a privately held medical technology company focused on accelerating the adoption of minimally invasive surgery (MIS) as the standard of care in spine, today announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its FlareHawk TiHawk™11 Interbody […]
Brainlab Announces the Majority Acquisition of medPhoton
MUNICH–(BUSINESS WIRE)–Brainlab, a digital medical technology company, announced today the majority acquisition of medPhoton GmbH, a Salzburg, Austria-based company, that develops and manufactures robotic imaging solutions for image guided radiation therapy and surgery. Over the last years, medPhoton has maintained a close partnership with Brainlab in the field of intraoperative imaging. This collaboration resulted in the […]
Safe Orthopaedics announces the installation of a second SORA unit in France and will accelerate the SORA program in the second quarter of 2022
Eragny-sur-Oise, France, May 12nd, 2022 08h45 CET – Safe (FR0013467123 – ALSAF), a company specializing in the design, manufacturing and marketing of single-use technologies for spinal surgeries, delivering the safest treatment for spinal fractures urgently treated, announces the installation of a second SORA unit in France and wishes to accelerate the SORA program. Safe Orthopaedics reminds the value […]
Globus Medical Reports First Quarter 2022 Results
AUDUBON, Pa., May 10, 2022 (GLOBE NEWSWIRE) — Globus Medical, Inc. (NYSE: GMED), a leading musculoskeletal solutions company, today announced its financial results for the first quarter ended March 31, 2022. Worldwide net sales were $230.5 million, an increase of 1.4% as compared to the first quarter of 2021 GAAP net income for the quarter was […]
Surgalign Holdings, Inc. Announces First Quarter 2022 Results and Raises Full Year 2022 Revenue Guidance Range
DEERFIELD, Ill., May 10, 2022 (GLOBE NEWSWIRE) — Surgalign Holdings, Inc., (NASDAQ: SRGA) a global medical technology company focused on elevating the standard of care by driving the evolution of digital surgery, today announced financial results for its 2022 first quarter ended March 31, 2022. Financial Highlights and Key Events: Received FDA 510(k) clearance for […]
SI-BONE, Inc. Reports First Quarter 2022 Financial Results
SANTA CLARA, Calif., May 09, 2022 (GLOBE NEWSWIRE) — SI-BONE, Inc. (Nasdaq:SIBN), a medical device company dedicated to solving musculoskeletal disorders of the sacropelvic anatomy, today reported financial results for the quarter ended March 31, 2022. Recent Highlights Worldwide revenue of $22.4 million for the first quarter 2022, representing a 10% increase over the corresponding period in 2021 U.S. revenue of $20.4 million for the […]
Mediliant Group Announces Matt Burba as Their Newly Appointed CEO
LE LOCLE, SWITZERLAND (PRWEB) MAY 10, 2022–Leaders in medical device contract manufacturing, the Mediliant Group, has today announced Matt Burba as the company’s new CEO. Poised for continuous advancement, the new leadership role of Mr. Burba is an important factor in the Mediliant Group’s expansion. Matt Burba came to the Mediliant Group as the top candidate […]
510(k) Clearance received for Anterior Cervical Plate System Aggeris-C.
Clariance Spine is proud to announce that Aggeris-C received 510(k) clearance. Aggeris-C is low profile anterior cervical plate system designed with the following features in mind: PRE-LORDOSED PLATEPre-lordosed plate conforms to the patient’s cervical vertebrae for enhanced stability. LOW PROFILELow profile to reduce the risk of contact with and irritation to soft tissues. SECURED PLATEVariable angle […]
Osseus Announces FDA 510(k) Clearance of the Pisces-SA Standalone ALIF Interbody System
DALLAS, TX, May 9, 2022 – Osseus, an innovative spinal solutions company, announces FDA 510(k) clearance and launch of the Pisces™-SA Standalone ALIF Interbody System. The Pisces™-SA interbody can be used with both bone screws and alternative fixation bone anchors allowing for increased intraoperative flexibility. Biomechanical testing proved that the Pisces™-SA anchors provide better expulsion resistance than the competition and perform comparably with traditional screw-based standalone ALIF constructs in stabilizing injured spinal segments. The Pisces™-SA is the first of its […]
CONMED Announces Definitive Agreement to Acquire In2Bones Global, Inc.
LARGO, Fla.–(BUSINESS WIRE)–May 4, 2022– CONMED Corporation (NYSE: CNMD) today announced a definitive agreement to acquire privately-held In2Bones Global, Inc. (In2Bones), on a cash-free, debt-free basis, for cash consideration of $145 million at closing and up to an additional $110 million in growth-based earnout payments over a four-year period. The transaction is not subject to a financing condition. The transaction is subject to […]
Variable-Dimension/Variable-Stiffness Spinal Fixation Rod with Bezier Surface Geometry Patent Approved
FORT LAUDERDALE, Fla.–(BUSINESS WIRE)–Subsequent to study results previously released by Spinal Resources Inc, the U.S. Patent and Trademark Office has issued the Variable-Dimension/Variable-Stiffness Spinal Fixation Rod with Bezier Surface Geometry a patent. The study demonstrated that Bezier-surface, spinal fixation rods provide quantifiable advantages over standard constant diameter rods, stepped-reduction-in-diameter rods and conical-shaped rods in assisting […]
OrthoSon completes £8.9m expanded Series A to accelerate back pain treatment’s progress to clinic
Oxford-based medical equipment firm OrthoSon, which is developing novel motion-restoring minimally invasive treatments for low back pain, has announced thecompletion of an £8.9 million Series A fund round to help prepare its product for Phase I trials in the US.New international investors in the expanded round include the Greek technology venture capital firm Big Pi Ventures […]
Spinal Simplicity Acquires Intralink-Spine
Overland Park, KS – May 4, 2022 – Spinal Simplicity, a medical device company, today announced the acquisition of Intralink-Spine and its novel injectable spinal disc stabilization product. The technology utilizes naturally derived compounds that self-polymerize and attach to collagen fibrils throughout the spinal disc. This process mechanically supports the disc to inhibit excessive motion and […]
ATEC Reports First Quarter 2022 Financial Results and Increases Full-Year Revenue Guidance
CARLSBAD, Calif.–(BUSINESS WIRE)– Alphatec Holdings, Inc. (Nasdaq: ATEC), a provider of innovative solutions dedicated to revolutionizing the approach to spine surgery, today announced financial results for the quarter ended March 31, 2022, and recent corporate highlights. First Quarter 2022 Financial Results First Quarter Highlights ATEC lateral procedures, including the Prone Trans-Psoas (PTP) Technique, delivered approximately 40% of revenue growth; […]
ZimVie Reports First Quarter 2022 Financial Results
WESTMINSTER, Colo., May 05, 2022 (GLOBE NEWSWIRE) — ZimVie Inc. (Nasdaq: ZIMV), a global life sciences leader in the dental and spine markets, today reported financial results for the quarter ended March 31, 2022. Management will host a corresponding conference call today, May 5, 2022 at 4:30 p.m. Eastern Time. “We are very pleased to […]
Accelus Launches TiHawk7™ Titanium-Bonded Multidirectionally Expanding Interbody Cage to Support Endoscopic and MIS Lumbar Fusion Procedures
PALM BEACH GARDENS, Fla., May 05, 2022 (GLOBE NEWSWIRE) — Accelus, a medical technology company focused on accelerating the adoption of minimally invasive surgery (MIS) as the standard of care in spine, today announced the alpha launch and first procedures performed utilizing its TiHawk7™ expandable interbody cage. TiHawk7 is the latest addition to Accelus’s FlareHawk® Interbody Fusion […]
Surgalign™ Announces the First Successful Surgical Procedure Utilizing its HOLO Portal™ Surgical Guidance System
DEERFIELD, Ill., May 05, 2022 (GLOBE NEWSWIRE) — Surgalign Holdings, Inc., (NASDAQ: SRGA) a global medical technology company focused on elevating the standard of care by driving the evolution of digital health, today announced that the Company’s HOLO Portal surgical guidance system has officially entered clinical use. The first procedure was performed at Indiana Spine […]
NuVasive Announces First Quarter 2022 Financial Results
SAN DIEGO, May 4, 2022 /PRNewswire/ — NuVasive, Inc. (NASDAQ: NUVA), the leader in spine technology innovation, focused on transforming spine surgery with minimally disruptive, procedurally integrated solutions, today announced financial results for the quarter ended March 31, 2022. First Quarter 2022 Net sales were $290.8 million, a 7.2% increase as reported and a 9.1% increase on a constant currency […]
SpineGuard’s DSG® Technology Applied to Robotics: Three Scientific Articles Presented at the Conference on New Technologies for Computer and Robot Assisted Surgery (CRAS)
SpineGuard (FR0011464452 – ALSGD), an innovative company that deploys its DSG® (Dynamic Surgical Guidance) sensing technology to secure and streamline the placement of bone implants, announced that research teams presented three articles at the CRAS conference on April 25th and 26th in Naples, Italy. They report the progress made with the robotic application of DSG. Stéphane Bette, co-founder […]
SeaSpine Reports 21 Percent Revenue Growth for First Quarter 2022 and Increases Full Year 2022 Revenue Guidance
CARLSBAD, Calif., May 03, 2022 (GLOBE NEWSWIRE) — SeaSpine Holdings Corporation (NASDAQ: SPNE), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, announced today financial results for the three-months ended March 31, 2022. Summary First Quarter 2022 Financial Results and Recent Highlights Total revenue of $50.7 million, an increase […]
SeaSpine® Announces New Senior Leadership Team Appointments
CARLSBAD, Calif., May 03, 2022 (GLOBE NEWSWIRE) — SeaSpine Holdings Corporation (NASDAQ: SPNE), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today announced that John Bostjancic, previously the Company’s Senior Vice President, Chief Financial Officer, has assumed expanded responsibilities and the title of Chief Operating and Financial Officer. […]
Wenzel Spine Announces Leadership Changes
AUSTIN, Texas–(BUSINESS WIRE)–Wenzel Spine, Inc., a medical technology company focused on providing minimally invasive surgical and diagnostic solutions for the treatment of spinal disorders, today announced that its Board of Directors has appointed Dr. Warren Neely as Chairman of the Board and William E. Wilson, President and Chief Executive Officer. On March 7th, 2022, the […]
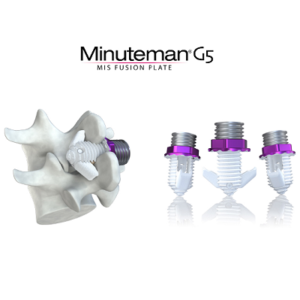
Spinal Simplicity Announces the launch of the Minuteman® G5 Implant
OVERLAND PARK, KAN. (PRWEB) MAY 03, 2022–Spinal Simplicity, a medical device company, today announced the much-anticipated launch of the Minuteman® G5 implant. The Minuteman® is a minimally invasive, interspinous-interlaminar fusion device intended for the fixation and stabilization of the thoracic, lumbar, and sacral spine while awaiting bony fusion to occur. It is designed for attachment to the […]