AUDUBON, Pa., Sept. 28, 2021 (GLOBE NEWSWIRE) — Globus Medical, Inc. (NYSE:GMED), a leading musculoskeletal solutions company, today announced it will participate in the 36th Annual Meeting of the North American Spine Society (NASS) being held September 29 through October 2, 2021 at the Boston Convention and Exhibition Center. Globus Medical will present the latest evolutions […]
2021
Intelivation Technologies Launches Golden Isles Pedicle Screw System™
SAINT SIMONS ISLAND, Ga., Sept. 28, 2021 /PRNewswire/ — Intelivation Technologies, a medical device company with a cutting-edge spinal implant product portfolio announced today the official launch of the Golden Isles Pedicle Screw System™ during the 36th Annual NASS Meeting in Boston September 29-Ocotber 2, 2021. The Golden Isles Pedicle Screw System™ is designed to provide immobilization and stabilization of spinal segments […]
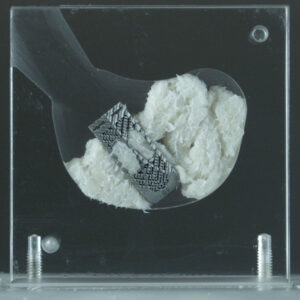
Spineology Launches First-of-Its-Kind Duo Ti™ Expandable Interbody Fusion Procedure
ST. PAUL, Minn.–(BUSINESS WIRE)–Spineology Inc., an innovator in anatomy-conserving surgery, is excited to launch the Duo Ti Expandable Interbody Fusion Procedure, which combines Spineology’s proprietary mesh technology with porous titanium to deliver a large, anatomy conforming implant via anatomy-conserving lateral decubitus and prone approaches. The porous titanium blocks are made from OsteoSync™ Ti (SITES Medical) and […]
Spine Wave Announces the Commercial Launch of the Salvo® 5.5/6.0mm Spine System
SHELTON, Conn., Sept. 27, 2021 (GLOBE NEWSWIRE) — Spine Wave is pleased to announce the successful completion of its limited market release and subsequent launch of the Salvo® 5.5/6.0mm Spine System featuring Spine Wave’s advanced modular screw design. Spine Wave launched the Salvo® 4.75mm Spine System during late 2020 to address cortical bone trajectory and […]
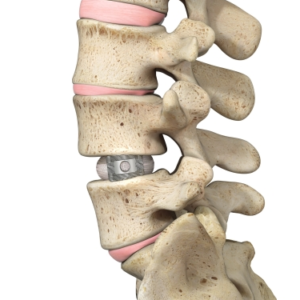
NGMedical GmbH Receives FDA Clearance for Its AM Titanium Lumbar Interbody BEE® PLIF
SCOTTSDALE, Ariz., Sept. 27, 2021 (GLOBE NEWSWIRE) — NGMedical, a German medical device manufacturer exclusively focused on creating innovative technologies for spinal application, announces FDA clearance for its purely additively manufactured titanium lumbar BEE® PLIF cage, which will be featured at NASS. The BEE® PLIF cage has been created to benefit from additive manufacturing features. The purposefully […]
SeaSpine Announces CE Mark Certification of 7D Surgical Cranial Module and Percutaneous Spine Module for Minimally Invasive Spine Surgery
CARLSBAD, Calif., Sept. 27, 2021 (GLOBE NEWSWIRE) — SeaSpine Holdings Corporation (NASDAQ: SPNE), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today announced it received simultaneous CE Mark certification for its Cranial Module and Percutaneous Spine Module for the 7D FLASH™ Navigation System. This achievement expands the applications […]
Kleiner Device Labs to Showcase New KG2 Surge Flow-Thru Interbody System at NASS
INCLINE VILLAGE, Nev., Sept. 27, 2021 /PRNewswire/ — Kleiner Device Labs today announced that at the upcoming NASS conference in Boston it will be showcasing the new KG™2 Surge™ flow-thru interbody system that recently received U.S. Food and Drug Administration (FDA) 510(k) market clearance. The company will have the KG2 Surge system for surgeons to handle and test with simulated […]
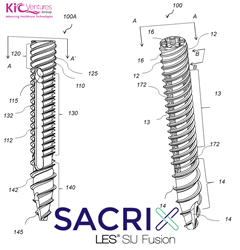
Sacrix LLC, A KICVentures Company, Published Study Showing Clinical And CT Scan Outcomes Of Its Percutaneous Lateral-Oblique Technique For Sacroiliac Joint (SIJ) Fusion
MALDEN, Mass., Sept. 24, 2021 /PRNewswire via COMTEX/ — MALDEN, Mass., Sept. 24, 2021 /PRNewswire-PRWeb/ — Sacrix LLC (Boston, MA) is pleased to announce the publication of the “Clinical Outcomes of Novel Lateral-Oblique Percutaneous Sacroiliac Joint (SIJ) Fixation“, a study using a technique developed by Dr. Kingsley R Chin MD, a Harvard-Trained Board-Certified Orthopedic Spine […]
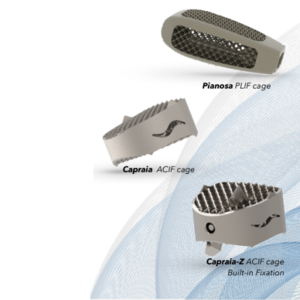
Tsunami Medical Announces Rebranding of ACIF &PLIF Cages:Capraia and Pianosa
Modena, September 27th 2021 Creativity is an essential fuel for the medical device industry, also when it comes to commercial product names. Through its commercial product names, Italy based Tsunami Medical has praised its heritage by conveying a sense of belonging and tribute to the country’s many beautiful islands. The company’s “wave of innovation” is […]
Intelivation Technologies Launches Advantage-C™ PEEK Cervical Interbody Fusion Device
SAINT SIMONS ISLAND, Ga., Sept. 24, 2021 /PRNewswire/ — Intelivation Technologies, a medical device company with a growing product portfolio announced today that they are officially launching the Advantage-C™ PEEK Interbody Fusion Device during the 36th Annual NASS Meeting in Boston September 29-October 2, 2021. The Advantage-C™ device is designed to be used in skeletally mature patients in levels C2-T1 in […]
Tyber Medical Reaches Agreement to Acquire CatapultMD
BETHLEHEM, Pa., Sept. 23, 2021 /PRNewswire/ — Since its founding in 2012, Tyber Medical has experienced record growth with its continually expanding product portfolio. In support of its rapid growth, Tyber implemented a vertical integration strategy, in 2019, that included a large-scale expansion of its headquarters located in the Lehigh Valley in Pennsylvania. As Tyber Medical continues to expand its customer […]
Implanet announces the planned divestment of its knee prosthesis to focus on its Spine business
Bordeaux, Boston, September 23, 2021 – 5.45 pm CEST – IMPLANET (Euronext Growth: ALIMP, FR0013470168, eligible for PEA-PME equity savings plans) a medical technology company specializing in vertebral and knee-surgery implants, announces the planned divestment of its knee prosthesis in order to devote itself to its Spine business. Implanet had announced in previous press releases, including that of […]
Medtronic Begins Pediatric Clinical Trial of Spinal Tether for Treatment of Scoliosis
DUBLIN, Sept. 22, 2021 /PRNewswire/ — Medtronic plc (NYSE: MDT), the global leader in medical technology, today announced that it has enrolled its first patient and completed the first surgical procedure in its BRAIVE™ IDE study, which will evaluate the safety and effectiveness of the Braive™ growth modulation system for treatment of progressive Adolescent Idiopathic Scoliosis (AIS). The first patient […]
National Institute of Health Awards SINTX Technologies Phase 1 Grant for SN-PEEK 3D Printed Spinal Implants
SALT LAKE CITY, Sept. 22, 2021 (GLOBE NEWSWIRE) — SINTX Technologies, Inc. (www.sintx.com) (NASDAQ: SINT) (“SINTX” or the “Company”), an original equipment manufacturer of advanced ceramics, announced today a Phase 1 grant of $308,301 awarded by the National Institute of Health (NIH) to develop a 3-D printed, composite silicon nitride – polyetheretherketone (SN-PEEK) spinal implant. This […]
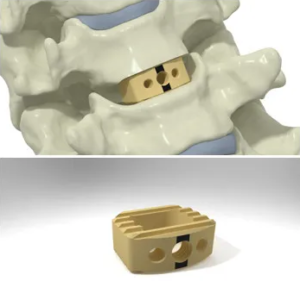
Spinal Elements® to Feature the Karma® Fixation System at Upcoming 36th Meeting of the North American Spine Society
Carlsbad, CA, September 22, 2021 – Spinal Elements, a spine technology company, today announced that the Karma Fixation System will be featured at the upcoming 36th Meeting of the North American Spine Society at the Boston Convention and Exhibit Center on September 29th through October 1st, 2021. The Karma system consists of a high-performance polymer fixation device […]
Camber Spine Launches SPIRA-P and SPIRA-T Implants for National Distribution
KING OF PRUSSIA, PA — September 22, 2021 – Camber Spine, a leading innovator in spine and medical technologies, has initiated the full national launch of its SPIRA-P Posterior Lumbar Spacer and SPIRA-T Oblique Posterior Lumbar Spacer devices. News of the national launch comes on the heels of Camber’s announcement last month that it has received U.S. Food and Drug Administration (FDA) 510(k) clearance […]
Kleiner Device Labs Receives FDA Market Clearance for KG2 SurgeFlow-Thru Interbody System
September 22, 2021 — Incline Village, Nev., — Kleiner Device Labs today announced that it has received U.S. Food and Drug Administration (FDA) 510(k) market clearance for its KGTM2 SurgeTMflow-thru interbodysystem. The system maximizes total bone graft delivery volume, better distributes graft bilaterally into the intervertebral disc space, and streamlines the implant delivery, positioning and […]
Astura Medical to Debut Several New Technology Platforms During the 2021 NASS Annual Meeting
IRVING, TX – September 22, 2021 – Astura Medical, a high-growth, innovative spine technology company, announced that it will be attending the 2021 North American Spine Society (NASS) Annual Meeting being held in Boston, MA from September 29th through October 1st. The Company’s current technology that is already widely recognized as best-in-class will be on […]
Life Spine to Showcase One of the Industry’s Largest Expandable Offerings and Full Spine Product Portfolio at NASS 2021
Huntley, IL, September 21, 2021 –Life Spine, a medical device company that designs, develops, manufactures and markets products for the surgical treatment of spinal disorders, announced today that it would be showcasing its complete line up of Micro Invasive procedural solutions, which includes 13 Expandable Interbody devices making it one of the largest and most […]
Horizon Technology Finance Leads $15 Million Venture Loan Facility to Spineology
FARMINGTON, Conn., Sept. 20, 2021 /PRNewswire/ — Horizon Technology Finance Corporation (NASDAQ: HRZN) (“Horizon”), a leading specialty finance company that provides capital in the form of secured loans to venture capital backed companies in the technology, life science, healthcare information and services, and sustainability industries, announced today it led a $15 million venture loan facility to Spineology, Inc. (“Spineology”), of which […]
AxioMed LLC, A KICVentures Group Portfolio Company, Achieved Landmark Patent For Lateral Lumbar Viscoelastic Disc Replacement Medical Device
MALDEN, MASS. (PRWEB) SEPTEMBER 18, 2021–AxioMed continues to press its advantages in the artificial total disc replacement market with its investment in lateral lumbar viscoelastic disc technology. At a time when revenues and margins are driving the spine industry, AxioMed chose to venture alone into developing a lateral lumbar viscoelastic disc replacement. We thank the United […]
Sacrix LLC To Be Issued A 3D Porous Patent On Its Threaded Implant And Lateral-Oblique Sacroiliac Joint Fusion Technique On September 28th, 2021
MALDEN, MASS. (PRWEB) SEPTEMBER 18, 2021–Vito Lore, the VP of innovations at KICVentures Group, said “the patent includes a device claim of our threaded implant with the fusion channel; also an embodiment with a 3D porous structure”. “Clinically the 3D porous structure is expected to enhance the threaded implant’s performance in fixating the sacroiliac joint and […]
CoreLink Surpasses 5,000 Entasis SI Joint Fusion Implantations With Patented Stackable Guide Wires
ST. LOUIS, Sept. 16, 2021 /PRNewswire/ — CoreLink, LLC, a leading designer and manufacturer of spinal implant systems, today announced the implantation of over 5,000 Entasis® Lateral SI Joint Fusion System compression screws to fuse the sacroiliac joint. CoreLink also announced the company has been awarded patent number 11,052,229 by the United States Patent and Trademark Office (USPTO). The patent, titled, […]
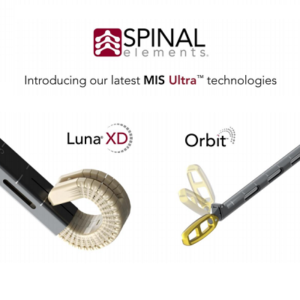
Spinal Elements® Announces Full Commercial Launch of Luna® XD and Orbit™ Systems
Carlsbad, CA, September 15, 2021 – Spinal Elements, a spine technology company, today announced the full commercial launch of the Luna XD multi-expandable lumbar interbody fusion device and Orbit articulating discectomy systems. Luna XD and Orbit have been integrated into Spinal Elements as the newest technologies in its MIS Ultra™ platform of products and procedural solutions. The […]
ATEC to Feature Comprehensive Procedural Solutions at NASS 2021
CARLSBAD, Calif.–(BUSINESS WIRE)– Alphatec Holdings, Inc. (Nasdaq: ATEC), a provider of innovative solutions dedicated to revolutionizing the approach to spine surgery, announced today that the Company’s comprehensive procedural portfolio will be featured at the North American Spine Society (NASS) Annual Meeting, which will be held at the Boston Convention and Exhibition Center from September 29 […]
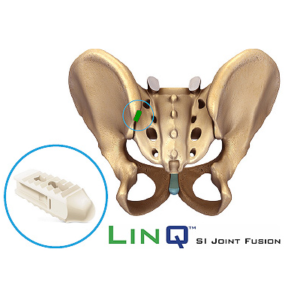
PainTEQ Acquires Three U.S. Patents for Innovative Sacroiliac (SI) Joint Procedure
TAMPA, Fla., Sept. 15, 2021 /PRNewswire/ –PainTEQ, a fast-growing,Tampa-based medical device company focused on treating sacroiliac (SI) joint dysfunction, announced the award of three new U.S. patents unique to the LinQ procedure. The LinQ™ process is a minimally invasive, novel procedure designed to help patients suffering from SI joint dysfunction. These patents solidify PainTEQ as an industry leader in minimally invasive […]
Aurora Spine Corporation Announces C$6.5 Million Private Placement with Institutional Investors
CARLSBAD, Calif., Sept. 15, 2021 (GLOBE NEWSWIRE) — Aurora Spine Corporation (“Aurora Spine” or the “Company”) (TSXV: ASG) (OTCQB: ASAPF), a designer and manufacturer of innovative medical devices that improve spinal surgery outcomes, today announced that it has entered into securities purchase agreements for a private placement of the Company’s common shares (or common share […]