SAN CARLOS, Calif., April 1, 2021 /PRNewswire/ — Empirical Spine, Inc., a company developing advanced solutions for the surgical treatment of spinal disorders, today announced that the US Food and Drug Administration (FDA) has granted Breakthrough Device Designation (BDD) for its LimiFlex™ Paraspinous Tension Band. In doing so, the FDA has determined that LimiFlex™ holds the potential […]
2021
UPDATED: Do you urgently need a surgical technique? Try here! We already have more than 725!
Today we have UPDATED the new section called BROCHURES where you can find 726 brochures and surgical techniques for the different products on the market. Now, everything is located in the same place.We believe that it is a very useful information when you want to find information about a product that is no longer on the market […]
SeaSpine® Appoints Google Executive and Healthcare Thought Leader to its Board of Directors
CARLSBAD, Calif., April 01, 2021 (GLOBE NEWSWIRE) — SeaSpine Holdings Corporation (NASDAQ: SPNE), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today announced that it has appointed Shweta Singh Maniar to its Board of Directors, effective April 1, 2021. Ms. Maniar is a Global Leader, Healthcare & Life […]
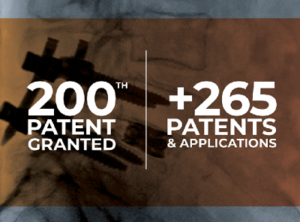
Life Spine Announces Issuance of 200th Patent
Huntley, IL, March 31st, 2021 –Life Spine, a leading medical device company that designs, develops, manufactures and markets products for the surgical treatment of spinal disorders, announced today that it has received notice of issuance from the U.S. Patent and Trademark Office (USPTO) regarding U.S. Patent No. 10,973,553, which signifies Life Spine’s 200th patent to date. […]
Premia Spine Announces FDA Breakthrough Device Designation for Its TOPS™ Spinal Arthroplasty System
NORWALK, Conn. – March31, 2021 – Premia Spine, a medical technology company aiming to change the way debilitating chronic leg and back pain is treated, today announced that its TOPS facet arthroplasty system won Breakthrough Device Designation from the U.S. Food & Drug Administration. TOPS is the first and only facet joint replacement system for […]
NGMedical GmbH Receives FDA Clearance for Its BEE® HA Cervical Cage System Made From PEEK-OPTIMATM HA Enhanced
SCOTTSDALE, Ariz., March 30, 2021 /PRNewswire/ — NGMedical, a German medical device manufacturer exclusively focused on creating innovative technologies for spinal application, announces FDA clearance for its anatomically shaped cervical interbody BEE® HA, made of the innovative PEEK-OPTIMA™ HA Enhanced from Invibio Biomaterial Solutions, a global leader in high-performance biomaterial solutions. BEE® HA was developed to optimize fusion while maintaining […]
Implanet finalizes the agreement to acquire OSD, a manufacturer of implants for spine surgery, to create a new benchmark player
Bordeaux, Boston, March 30, 2021 – 5.45 pm CEST: IMPLANET (Euronext Growth: ALIMP, FR0013470168, eligible for PEA-PME equity savings plans), a medical technology company specializing in vertebral and knee-surgery implants, today announces that it has finalized the agreement to acquire Orthopaedic & Spine Development (“OSD”), under suspensive conditions, which specializes in developing, manufacturing and marketing implants […]
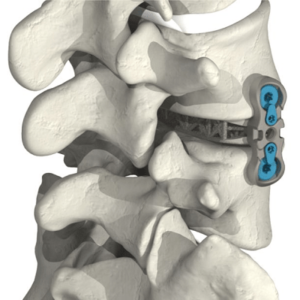
SpineVision is proud to announce the first Hexanium ACIF successfully implanted in the U.S.
SpineVision is proud to announce the first Hexanium ACIF successfully implanted in the U.S.We would like to thank Dr. Ripul R. Panchal from the American Neurospine Institute in Texas, for his expertise in using the Honeycomb 3D printed standalone cervical cage. The Hexanium ACIF cage is a 3D printed standalone cervical interbody system made of […]
Dr. Timothy Deer joins PainTEQ Scientific Advisory Board
TAMPA, Fla., March 30, 2021 /PRNewswire/ — Medical device innovator PainTEQ announced the appointment of Timothy Deer, MD, DABPM, to its Scientific Advisory Board. Deer is president and CEO of The Spine & Nerve Centers in Charleston, West Virginia. PainTEQ is the creator of LinQ (pronounced “link”), a minimally invasive therapy for sacroiliac (SI) joint dysfunction. With extensive experience in interventional pain management, […]
International development strategy pursuit in Asia and in the United States
Ecully, 29 March 2021 – Continued international development Workshop in Thailand dedicated to Spineway implants and instruments Signature of a distribution agreement in the United States Spineway, specialized in the design of innovative surgical implants and instruments for the treatment of severe disorders of the spine, continues its international development with the organization of a […]
Aurora Spine receives IRB approval for Multicenter Study of its ZIP® Interspinous Fixation Device for Relief of Back Pain
CARLSBAD, Calif., March 29, 2021 (GLOBE NEWSWIRE) — Aurora Spine Corporation (“Aurora Spine” or the “Company”) (TSXV: ASG) (OTCQB: ASAPF), a designer and manufacturer of innovative medical devices that improve spinal surgery outcomes, today announced that it has received Institutional Review Board (IRB) approval for its new multicenter study of its ZIP® Interspinous Fixation device for […]
UPDATED II: +50 Corpectomy Cages to Know!
Hello, today we are republishing the post regarding the list of “Corpectomy products to know.” Unfortunately, we missed some expandable systems (KONG,SAMSON, VALEO VBR). We are sorry about that. We thank Icotec and HumanTech for contacting us. Whenever we publish we try not to miss any product. Since the spine market is wide with many […]
SpineGuard reports the end of its French ‘sauvegarde’ proceeding
Paris (France) and Boulder (CO, USA), March 26, 2021 – SpineGuard (FR0011464452 – ALSGD), an innovative company that deploys its DSG® (Dynamic Surgical Guidance) sensing technology to secure and streamline the placement of bone implants, reported the end of its French ‘sauvegarde’ proceeding following the hearing held on March 10, 2021 at the Commercial Court of […]
Aurora Spine Receives FDA 510(K) Clearance for Its Proprietary APOLLO Anterior Cervical Plate
CARLSBAD, Calif., March 25, 2021 (GLOBE NEWSWIRE) — Aurora Spine Corporation (“Aurora Spine” or the “Company”) (TSXV: ASG) (OTCQB: ASAPF), a designer and manufacturer of innovative medical devices that improve spinal surgery outcomes, announced today that it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its proprietary APOLLO Anterior Cervical Plate (ACP) […]
Spineway: News Business Agreement dedicated to 3D innovation
Spineway announces the signature of a partnership with the Italian company Tsunami Medical, pioneer in innovative 3D-printed solutions, for the sale of Twin Peaks interbody cages produced with 3D printers. Always with the goal in mind of designing state-of-the-art technological solutions to meet the needs of surgeons for the treatment of spinal disorders, Spineway and Tsunami Medical […]
Inspired Spine Hosts OLLIF Observation and Cadaver Lab
BURNSVILLE, Minn., March 23, 2021 /PRNewswire/ — As its standard practice to employ the most advanced training methods available, Inspired Spine hosted a Surgery Observation and Cadaver Lab last week at its Burnsville campus. Doctors from across the country attended this event to observe two live OLLIF procedures and receive hands-on training for this technique. The participants also witnessed […]
ATEC Announces Memphis Distribution Facility
CARLSBAD, Calif., March 23, 2021 (GLOBE NEWSWIRE) — Alphatec Holdings, Inc. (Nasdaq: ATEC), a provider of innovative solutions dedicated to revolutionizing the approach to spine surgery, announced today the intention to lease a 75,000 square foot facility in Memphis, Tennessee, which will house ATEC’s primary distribution operations. The new facility, from which ATEC will distribute […]
OrthoPediatrics Surpasses Milestones of 500 Scoliosis Correction Cases Worldwide and Nine-Years of Clinical Experience with the ApiFix Procedure
WARSAW, Ind., March 23, 2021 (GLOBE NEWSWIRE) — OrthoPediatrics Corp. (“OrthoPediatrics” or the “Company”) (Nasdaq:KIDS), a company focused exclusively on advancing the field of pediatric orthopedics, today announced that it has surpassed two significant procedure milestones for its ApiFix®technology – an exciting alternative for the treatment of progressive adolescent idiopathic scoliosis (AIS). Over 500 ApiFix procedures have […]
Fusion Robotics™ Completes First Spine Procedure
MEMPHIS, Tenn., March 22, 2021 /PRNewswire/ — Fusion Robotics LLC, a spinal robotics and navigation company today announced the successful completion of the first spine procedure using the company’s technology platform. The procedure, completed by Dr. Kevin Foley at Baptist Memorial Hospital in Memphis TN, was a single-level minimally invasive transforaminal lumbar interbody fusion (MI-TLIF), including the placement of pedicle screw […]
SeaSpine Announces Agreement to Acquire 7D Surgical
CARLSBAD, Calif., March 22, 2021 (GLOBE NEWSWIRE) — SeaSpine Holdings Corporation (NASDAQ: SPNE), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, announced today that it has entered into an agreement to acquire all of the issued and outstanding shares of 7D Surgical, Inc., a privately-held, Toronto-based company, in […]
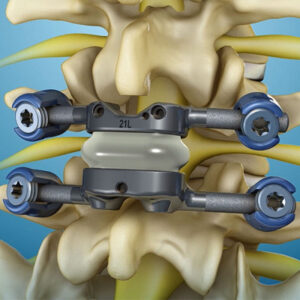
Life Spine Announces FDA 510(k) Clearance for the PROLIFT® Wedge Expandable Spacer System
Huntley, IL, March 18th, 2021 –Life Spine, a leading medical device company that designs, develops, manufactures and markets products for the surgical treatment of spinal disorders, announced today that it has received clearance from the U.S. Food & Drug Administration (FDA) to market the PROLIFT Wedge Expandable Spacer System. The PROLIFT Wedge Expandable Interbody Spacer […]
Exclusive negotiations for the acquisition of an European spinal entity
Spineway, specialized in innovative implants for the treatment of severe disorders of the spinal column (spine), announces that it has entered exclusive negotiations with a view to the acquisition of 100% of the capital of a European start-up specialized in the design, manufacture and sale of implants and instruments for spinal surgery. With its founder having […]
Implanet confirms offer to acquire a majority stake in Orthopaedic & Spine Development
IMPLANET (Euronext Growth: ALIMP, FR0013470168, eligible for PEA-PME equity savings plans), a medical technology company specializing in vertebral and knee-surgery implants, today announces that it has obtained founding shareholders’ agreement to acquire a majority stake in Orthopaedic & Spine Development (“OSD”), which specializes in developing, manufacturing and marketing implants for spine surgery. IMPLANET’s offer is […]
Camber Spine Launches National Rollout of Complete Device System to Support OLIF Technique
KING OF PRUSSIA, PA — March 16, 2021 – Camber Spine, a leading innovator in spine and medical technologies, has announced the national rollout of a complete set of devices and tools required to perform the Oblique Lateral Interbody Fusion Surgery (OLIF) technique. Camber’s OLIF technology platform, a continuation of the company’s ongoing portfolio of product development of surgical solutions […]
4WEB Medical Receives 510(k) Clearance for its Lumbar Plating Solution
DALLAS, March 17, 2021 /PRNewswire/ — 4WEB Medical, an orthopedic device company focused on developing innovative implants with an Advanced Structural Design utilizing its proprietary Truss Implant Technology™, announced today that the company has received 510(k) clearance from the U.S. Food & Drug Administration to market its Lumbar Plating Solution (LSTS-PS). The new plating solution consists of a […]
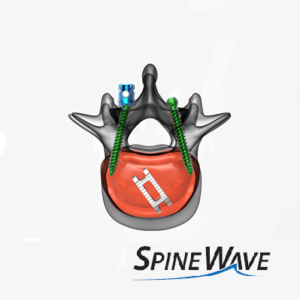
Spine Wave Announces the Commercial Launch of the Stronghold™ 3D Titanium Interbody Device
SHELTON, Conn., March 16, 2021 (GLOBE NEWSWIRE) — Spine Wave is pleased to announce the commercial launch of the Stronghold™ 3D Titanium Interbody Device featuring TiCell™ 3D advanced surface technology. This exciting new titanium lumbar interbody fusion implant is manufactured using a direct metal laser sintering manufacturing process (3D printing) to produce a unique surface […]
Surgalign Holdings, Inc. Announces Fourth Quarter and Full Year 2020 Results and Introduces 2021 Guidance
DEERFIELD, Ill., March 16, 2021 (GLOBE NEWSWIRE) — Surgalign Holdings, Inc. (Nasdaq: SRGA), a global medical technology company focused on advancing spine surgery and improving patient outcomes, including through the application of digital technology, today reported operating results for the fourth quarter and full year 2020. Recent Highlights: Total fourth quarter 2020 global spine revenue […]