Thank you Tsunami Medical ! On behalf of the SPINEMarketGroup team, we would like to thank you for supporting our site in 2021 through your PLATINUM sponsorship. About Tsunami Medical The company has been founded in 1997 as a subcontractor of some big manufacturing companies of invasive diagnostic devices. Over the years we bought the […]
2021
SeaSpine Reports First Quarter 2021 Financial Results
CARLSBAD, Calif., May 03, 2021 (GLOBE NEWSWIRE) — SeaSpine Holdings Corporation (NASDAQ: SPNE), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today announced financial results for the three-months ended March 31, 2021. Summary of First Quarter 2021 Financial Results and Recent Accomplishments Total revenue of $42.0 million, reflecting […]
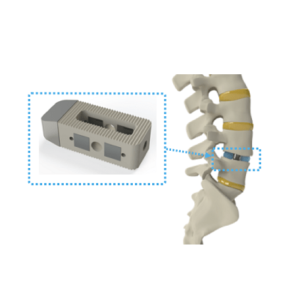
Intelligent Implants receives FDA breakthrough device designation for SmartFuse technology
Intelligent Implants Ltd. has announced FDA breakthrough device designation for its SmartFuse orthopedic implant technology, according to a press release. The SmartFuse platform is designed to remotely stimulate, control and monitor bone growth for real-time clinical decision-making. The product will be indicated for first-use patients who undergo lumbar spinal fusions, according to the release. “We […]
Stryker reports first quarter 2021 operating results
Kalamazoo, Michigan, April 27, 2021 (GLOBE NEWSWIRE) — Stryker (NYSE:SYK) reported operating results for the first quarter of 2021: The response to the COVID-19 pandemic has included measures to slow the spread of the virus taken by governments and health care authorities globally, including the postponement of elective medical procedures and social contact restrictions. While […]
Life Spine Announces 23% Revenue Growth in their Micro Invasive Expandable Lateral Spacer Systems
Huntley, IL, April 27th, 2021 –Life Spine, a leading medical device company that designs, develops, manufactures, and markets products for the surgical treatment of spinal disorders, announced today a 23% revenue growth in their Micro Invasive Expandable Lateral Spacer Systems from Q1 2021 over Q4 2020. Life Spine is the leader in Micro Invasive expandable […]
We are proud to announce that Clariance Spine is Sponsor of SPINEMarketGroup in 2021!
Thank you Clariance Spine ! On behalf of the SPINEMarketGroup team, we would like to thank you for supporting our site in 2021 through your PLATINUM sponsorship. About Clariance Spine Clariance is a French company specialized in spine surgery. Since 2007, we have been developing solutions with excellence that improve clinical outcomes.For more information, please […]
FDA grants Clariance Spine 510k clearance for the New Lateral Cage IDYS®-LLIF 3DTI
Clariance is proud to announce that Idys-LLIF 3DTi received 510(k) clearance earlier this month. Idys-LLIF 3DTi is a lateral lumbar interbody system designed with the following features in mind: STRONG FIXATIONConcave plate design with a 2-screw configuration provides a wide range of angulation for optimal cortico-cancellous bone purchase. EASY SCREW INSERTIONUnique plate design facilitates an […]
Curiteva Adds Steve Cichy as Strategic Advisor and Erik Erbe, PhD, as Vice President of Clinical and Research
HUNTSVILLE, Ala., April 28, 2021 /PRNewswire/ — Curiteva, Inc. announced today the addition of Steve Cichy as a Strategic Advisor to the rapidly growing technology company headquartered in Huntsville, Alabama. Cichy is an industry veteran who most recently led the Titan Spine sales organization for 13 years through a successful acquisition by Medtronic in 2019. “I recognized Curiteva as a […]
Camber Spine Pledges Support to Building Up the Youth
KING OF PRUSSIA, PA — April 27, 2021 – Camber Spine, a leading innovator in spine and medical technologies, has joined forces with Building Up The Youth, a local-area non-profit organization. Camber has pledged its support of the organization and its inner city youth assistance and training programs with donations, Camber staff volunteer hours and marketing and fundraising assistance. Camber […]
Orthofix Announces FDA Clearance and First Patient Implant of the 3D-Printed FORZA Titanium TLIF Spacer System with Nanovate Technology
LEWISVILLE, Texas–(BUSINESS WIRE)– Orthofix Medical Inc. (NASDAQ:OFIX), a global medical device company with a spine and extremities focus, today announced the U.S. Food and Drug Administration 510(k) clearance and first patient implant of the FORZA™ Ti TLIF Spacer System. Developed to help optimize Transforaminal Lumbar Interbody Fusion (TLIF) procedures, the FORZA Ti Spacer with Nanovate™ Technology is a titanium […]
Spinal Elements® Announces FDA 510(k) Clearance of Lucent® 3D Additive Manufactured Interbody Devices
Carlsbad, CA – April 20, 2021 – Spinal Elements, a spine technology company, today announced the first 510(k) clearance in the Lucent® 3D line of 3D-printed interbody devices. In developing Lucent 3D, Spinal Elements has taken advantage of the unique capabilities of 3D printing by printing a functionally unique multi-component device in a single printing […]
SpineGuard Strengthens Its DSG® Technology With the Validation of French and European Patents About Bone Quality Measurement
Paris (France) and Boulder (CO, USA), April 20, 2021 – SpineGuard (FR0011464452 – ALSGD), an innovative company that deploys its DSG® (Dynamic Surgical Guidance) sensing technology to secure and streamline the placement of bone implants announced today the intention to grant received from the French and European Patent Offices regarding patents describing the use of DSG technology to […]
SeaSpine Announces Limited Commercial Launch of the WaveForm® TO (TLIF Oblique) 3D-Printed Interbody System
CARLSBAD, Calif., April 19, 2021 (GLOBE NEWSWIRE) — SeaSpine Holdings Corporation (NASDAQ: SPNE), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today announced the limited commercial launch of its 3D-printed WaveForm TO (TLIF Oblique) Interbody System. WaveForm TO was designed for both PLIF (posterior lumbar interbody fusion) and […]
Globus Medical Announces First Surgeries with Revolutionary CREO ONE™ Robotic Screw
AUDUBON, Pa., April 15, 2021 (GLOBE NEWSWIRE) — Globus Medical, Inc. (NYSE: GMED), a leading musculoskeletal solutions company, today announced the recent launch of CREO ONE™, the market’s first robotic screw designed for spine surgery with ExcelsiusGPS®. CREO ONE™ enhances the company’s groundbreaking modular pedicle screw platform by simplifying pedicle preparation while maintaining navigational accuracy and increasing pullout strength by 86% compared to traditional pedicle screws tapped to size. The screw […]
SpineGuard Strengthens Its US Organization
Paris (France) and Boulder (CO, USA), April 15, 2021 – SpineGuard (FR0011464452 – ALSGD), an innovative company that deploys its DSG® (Dynamic Surgical Guidance) sensing technology to secure and streamline the placement of bone implants announced today the appointment of Patrick Pilcher as Vice President of Sales & Marketing for the United States, effective May 3, 2021. […]
Surgalign Appoints Marc Mackey as Executive Vice President, Digital Surgery
DEERFIELD, Ill., April 14, 2021 (GLOBE NEWSWIRE) — Surgalign Spine Technologies, (NASDAQ: SRGA) a global medical technology company focused on elevating the standard of care by driving the evolution of digital surgery, today announced the appointment of Marc Mackey as Executive Vice President, Digital Surgery, effective immediately. In the newly created position reporting directly to […]
Biogennix Launches Agilon Strip Advanced Bone Grafting Solution
IRVINE, Calif. — April 14, 2021 — Irvine-based Biogennix, an osteobiologics company that develops, manufactures, and distributes proprietary bone graft products used for bone fusion procedures, today announced the launch of its new Agilon® Strip bone grafting product. Developed with Biogennix’s proprietary TrelCorTM technology, Agilon Strip is the first collagen strip product introduced by Biogennix. Agilon Strip rounds out the company’s […]
Amplify Surgical®, Inc. Announces 1000th Level Treated with dualX® Dual Expanding Interbody Fusion Implant
LAGUNA HILLS, CALIF. (PRWEB) APRIL 13, 2021 Amplify Surgical, Inc., a medical device company focused on innovative dual-expanding technology for the lumbar spine, is pleased to announce that the company reached the milestone of treating 1000 spinal levels with the dualX Dual Expanding Interbody Fusion System. This noteworthy accomplishment was performed by Dr. Pawel Jankowski from […]
Spineology Adds Experienced Sales Executives to Sales Leadership Team
ST. PAUL, Minn.–(BUSINESS WIRE)–Spineology Inc., an innovator in anatomy-conserving surgery, welcomes two new area vice presidents to its sales leadership team. With proven track records in the spine industry, Jim Duffy will be Area Vice President of U.S. Sales, East, and Scott Sieczkarek will be Area Vice President of U.S. Sales, West. Duffy and Sieczkarek […]
SpineGuard Announces Its Full-Year 2020 Financial Results, First Quarter 2021 Sales and a Flexible Financing of €10M
PARIS and BOULDER (CO), April 8, 2021 – 17h45 CEST – SpineGuard (FR0011464452 – ALSGD), an innovative company that deploys its DSG® (Dynamic Surgical Guidance) sensing technology to secure and streamline the placement of bone implants announced today its full-year 2020 financial results, as approved by the Board of Directors on April 8, 2021, its […]
We are proud to announce that Viktor Hegedüs GmbH is Sponsor of SPINEMarketGroup in 2021!
Thank you Viktor Hegedüs GmbH ! On behalf of the SPINEMarketGroup team, we would like to thank you for supporting our site in 2021 through your PLATINUM sponsorship. About Viktor Hegedüs GmbH The name Viktor Hegedüs has stood for extraordinary innovations and high-quality precision in the fields of medical technology and industrial applications for many years […]
ATEC Announces Preliminary First Quarter 2021 Revenue Results and Provides Corporate Updates
CARLSBAD, Calif., April 08, 2021 (GLOBE NEWSWIRE) — Alphatec Holdings, Inc. (Nasdaq: ATEC), a provider of innovative solutions dedicated to revolutionizing the approach to spine surgery, announced today preliminary revenue results for the first quarter ended March 31, 2021. The Company confirmed that the Autorité des marchés financiers (AMF), France’s financial markets regulator, cleared ATEC’s […]
HT Medical Announces Name Change to Xēnix Medical as Part of Rebranding Initiative
ORLANDO, Fla.–HT Medical, a surgical implant company focused on the development of novel science-based solutions for patients requiring spinal fusion surgery, today announced that it has changed its name to Xēnix Medical as part of a rebranding initiative. The company also revealed that it has moved its corporate office to an expanded location in order […]
We are proud to announce that PRODORTH is Sponsor of SPINEMarketGroup in 2021!
Thank you PRODORTH ! On behalf of the SPINEMarketGroup team, we would like to thank you for supporting our site in 2021 through your PLATINUM sponsorship. Prodorth Spine RD Medical is dedicated to provide the best spinal implants&instruments to the market as it’s founded and supported by mechanical and materials engineers who are experienced in […]
Life Spine, Inc. Announces Preliminary Injunction Prohibiting Sale of Products Found To Be Reverse Engineered From Its ProLift® Expandable Cage
HUNTLEY, IL – April 6th, 2021–A preliminary injunction preventing the marketing and sale of Aegis Spine, Inc.’s AccelFix-XT line of expandable medical devices is now in effect. The United States District Court for the Northern District of Illinois issued the injunction after finding that Aegis and its parent company, L&K Biomed Co. Ltd., used Life […]
NuVasive’s Simplify Disc Receives FDA Approval for Two-Level Cervical Total Disc Replacement
SAN DIEGO, April 6, 2021 /PRNewswire/ — NuVasive, Inc. (NASDAQ: NUVA), the leader in spine technology innovation, focused on transforming spine surgery with minimally disruptive, procedurally integrated solutions, today announced the NuVasive Simplify® Cervical Artificial Disc (Simplify Disc) received approval from the U.S. Food and Drug Administration (FDA) for two-level cervical total disc replacement (cTDR). “This approval is an incredible […]
SeaSpine Announces Preliminary Results for First Quarter 2021 and Increases 2021 Revenue Guidance
CARLSBAD, Calif., April 05, 2021 (GLOBE NEWSWIRE) — SeaSpine Holdings Corporation (NASDAQ: SPNE), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, announced today preliminary financial results for the first quarter of 2021 and provided updated revenue guidance for the full-year 2021. The Company also provided additional information regarding […]