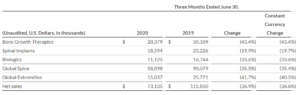
COVID 2019 has caused this year to be quite complicated for the spine market. According to Reportlinker.com, the global spine implants market is expected to decline from $12.44 billion in 2019 to $11.83 billion in 2020 at a compound annual growth rate (CAGR) of -4.92%. The decline is mainly due to the COVID-19 outbreak that has […]
2020
Xtant Medical Announces U.S. Commercial Launch of the Matriform® Si for Spinal Fusion Procedures
BELGRADE, Mont., Aug. 24, 2020 (GLOBE NEWSWIRE) — Xtant Medical Holdings, Inc. (NYSE American: XTNT), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today announced the U.S. commercial launch of the Matriform® Si, a silicated synthetic bone graft strip designed and cleared for spinal fusion procedures. The Matriform […]
Atlas Spine Announces Launch of its HiJAK SA Expandable Cervical Stand-Alone Interbody System
JUPITER, FL, August 19, 2020 — Atlas Spine, Inc., a spinal implant company based in Jupiter Florida, announced today the launchof its HiJAK SA Expandable Cervical Stand-Alone Interbody system, the company’s second first-to-market expandable system in just 18 months. The first implantation of the HiJAK SA Expandable Stand–Alone was performed by board certified neurosurgeon, Grant […]
Aurora Spine Receives “Notice of Allowance” for Patent: DEXA Technology™ —The World’s First Patient-Matched Interbody Implant Technology to Enable Improved Spine Surgery
CARLSBAD, Calif., Aug. 18, 2020 (GLOBE NEWSWIRE) — Aurora Spine Corporation (“Aurora Spine” or the “Company”) (TSXV: ASG) today announced it has received “Notice of Allowance” for its patent from the United States Patent and Trademark Office (USPTO) for Patient-Matched Interbody Implant Technology. This patent will be utilized to create spinal implants that match the […]
Surgalign Names Douglas S. Bireley, Executive Vice President, Marketing and Research & Development
DEERFIELD, Ill., Aug. 17, 2020 (GLOBE NEWSWIRE) — Surgalign Holdings, Inc. (Nasdaq: SRGA), a leading global pure-play spine company focused on advancing spine surgery and improving patient outcomes, today announced the appointment of Douglas S. Bireley as Executive Vice President, Marketing and Research & Development, effective August 17, 2020. Bireley joins Surgalign with over 20 […]
Centinel Spine Announces Varun Gandhi as Chief Financial Officer
WEST CHESTER, Pa., Aug. 17, 2020 /PRNewswire/ — Centinel Spine®, LLC, the largest privately-held spine company focused on anterior column reconstruction, today announced Varun Gandhi as Chief Financial Officer effective August 17, 2020. Mr. Gandhi succeeds Jeff Stello of Charlestown Capital Partners, who had served as interim CFO since late 2019. Varun Gandhi was most recently Senior Vice President of Corporate Finance and […]
Fuse Medical, Inc. Files Quarterly Results on Form 10-Q and Provides Business Update in response of COVID-19
RICHARDSON, TX, August 10, 2020 /Businesswire/ — Fuse Medical, Inc. (OTCPINK: FZMD) (“Fuse” or the “Company”) announced that it filed its Quarterly Report on Form 10-Q for the fiscal quarter ended June 30, 2020 with the Securities and Exchange Commission (“SEC”) on Friday, August 7, 2020. The COVID-19 pandemic posed an unprecedented challenge to the […]
Life Spine announces FDA 510(k) clearance for the PLATEAU®-X TI Lateral Lumbar Spacer System
Huntley, IL, August 11th, 2020 –Life Spine, a medical device company that designs, develops, manufactures and markets products for the surgical treatment of spinal disorders, announced today that it has received clearance from the U.S. Food & Drug Administration (FDA) to market the PLATEAU-X Ti Lateral Lumbar Spacer System. “The launch of PLATEAU-X Ti furthers […]
SeaSpine® Announces Limited Commercial Launch of Explorer™ TO Expandable Interbody Device
CARLSBAD, Calif., Aug. 11, 2020 (GLOBE NEWSWIRE) — SeaSpine Holdings Corporation (NASDAQ: SPNE), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today announced the limited commercial launch of the Explorer™ TO System. Explorer TO represents SeaSpine’s first new product offering since 2017 in the estimated $400 million expandable interbody […]
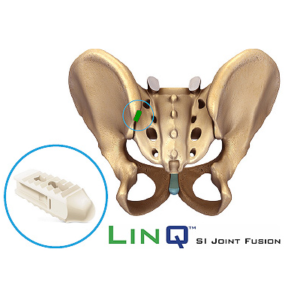
PainTEQ Begins Novel Biomechanical Study on SI Joint Fusion
TAMPA, Fla., Aug. 11, 2020 /PRNewswire/ — PainTEQ, a leading medical device innovator, launched a first of its kind biomechanical study this month that explores the treatment of sacroiliac (SI) joint pain through a minimally invasive outpatient procedure using a posterior approach. The study will explore the function and motion of test subjects’ bilateral joints that have been treated […]
Safe Orthopaedics receives 510k approval from the FDA for the sale of its 2nd Generation of SteriSpineTM PS in the United States
Eragny-sur-Oise, France, August 10th à 6.00 PM CEST – Safe Orthopaedics (FR0013467123 – ALSAF), a company specializing in the design and marketing of ready-to-use technologies for spinal surgeries, delivering the safest treatment of spinal fractures urgently treated, is annoucing today the 510k approval from the American Regulatory Agency Food and Drug Administration (FDA), for the sale […]
Xtant Medical Announces Debt Restructuring
BELGRADE, Mont., Aug. 10, 2020 (GLOBE NEWSWIRE) — Xtant Medical Holdings, Inc. (NYSE American: XTNT), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today announced that it has entered into a Restructuring and Exchange Agreement with the lenders under its credit facility pursuant to which the parties agreed to […]
LES Spine Innovations announces launch of the new A-CIFT SoloFuse HYPER-LORDOTIC Implant offerings
BOSTON (PRWEB) AUGUST 08, 2020 LES Spine Innovations and Sagitechnology are proud to announce the launch of the new A-CIFT SoloFuse Hyperlordotic Cervical Standalone Interbody System, the first ever HA impregnated standalone cervical cage for greater sagittal correction. The A-CIFT SoloFuse Hyperlordotic Interbody is a Less Exposure Surgery (LES®) technology. LES technologies are designed with outpatient surgery in […]
Surgalign Holdings, Inc. Announces Second Quarter 2020 Results
DEERFIELD, Ill., Aug. 07, 2020 (GLOBE NEWSWIRE) — Surgalign Holdings, Inc. (Nasdaq: SRGA), a leading global pure-play spine company focused on advancing spine surgery and improving patient outcomes, today reported operating results for the second quarter of 2020. The sale of RTI Surgical Holdings, Inc.’s (“RTI”) OEM business closed on July 20, 2020, accordingly, the […]
Orthofix Reports Second Quarter 2020 Results
LEWISVILLE, Texas–(BUSINESS WIRE)–Aug. 6, 2020– Orthofix Medical Inc. (NASDAQ:OFIX) today reported its financial results for the second quarter ended June 30, 2020. Net sales were $73.1 million, earnings per share (“EPS”) was ($0.96) and adjusted EPS was ($0.59). “Despite the significant impact of COVID-19 in the second quarter, we continued to execute successfully against our strategic initiatives,” said Orthofix President and […]
ATEC Reports Second Quarter 2020 Financial Results and Recent Corporate Highlights
August 06, 2020 16:05 ET | Source: Alphatec Holdings, Inc. • U.S. Revenue Grows 11%• Seventh Consecutive Quarter of Year-Over-Year Double-Digit Revenue Increase CARLSBAD, Calif., Aug. 06, 2020 (GLOBE NEWSWIRE) — Alphatec Holdings, Inc. (“ATEC” or the “Company”) (Nasdaq: ATEC), a provider of innovative spine surgery solutions dedicated to revolutionizing the approach to spine surgery, today announced financial results for the quarter […]
Globus Medical Reports Second Quarter 2020 Results
AUDUBON, Pa., Aug. 05, 2020 (GLOBE NEWSWIRE) — Globus Medical, Inc. (NYSE: GMED), a leading musculoskeletal solutions company, today announced its financial results for the second quarter ended June 30, 2020. Worldwide sales were $148.9 million, a decrease of 23.4% as reported Second quarter net loss was $20.8 million Diluted earnings per share (EPS) was $(0.21) and non-GAAP […]
Life Spine Announces FDA 510(k) Clearance for the for the PLATEAU®-A TI Anterior Lumbar Spacer System
Huntley, IL, August 5 , 2020 –Life Spine, a medical device company that designs, develops, manufactures and markets products for the surgical treatment of spinal disorders, announced today that it has received clearance from the U.S. Food & Drug Administration (FDA) to market the PLATEAU-A Ti Anterior Lumbar Spacer System. “With the increased usage of […]
WishBone Medical Announces Sterile Spine Offerings With New Acquisitions
WARSAW, Ind., Aug. 05, 2020 (GLOBE NEWSWIRE) — WishBone Medical Inc., pediatric orthopedic company focused globally on the unmet needs of children suffering from orthopedic issues, today announced its 5th and 6th strategic acquisition in the past 18 months by acquiring substantially all assets of Back 2 Basics Direct (B2B) and Orbbö Surgical, two privately-held medical device […]
SACRIX, LLC Announces Successful Adoption of Its Revolutionary Bloodless Sacroiliac Fixation Technology At World Leading Academic Center In The USA
BOSTON (PRWEB) AUGUST 04, 2020–Sacrix, LLC, a KICVentures Company, invented a new 100% percutaneous posterior sacroiliac joint (SIJ) fixation technique that can be done in less than 20 minutes through a 1.5cm incision in an outpatient setting with no blood loss. This new technique for outpatient SIJ fusion uses a pair of patented FDA-cleared threaded cannulated […]
NuVasive Announces Second Quarter 2020 Financial Results
SAN DIEGO, Aug. 4, 2020 /PRNewswire/ — NuVasive, Inc. (NASDAQ: NUVA), the leader in spine technology innovation, focused on transforming spine surgery with minimally disruptive, procedurally integrated solutions, today announced financial results for the quarter ended June 30, 2020. Second Quarter 2020 Highlights Net sales decreased -30.3% to $203.6 million, or -30.2% on a constant currency basis; GAAP operating margin of […]
SeaSpine® Reports Second Quarter 2020 Financial Results
CARLSBAD, CA (August 4, 2020) – SeaSpine Holdings Corporation (NASDAQ: SPNE), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today announced financial results for the three months ended June 30, 2020. Summary of Second Quarter 2020 Financial Results and Recent Accomplishments Revenue of $28.6 million, a decrease of 27% […]
Zimmer Biomet Announces Second Quarter 2020 Financial Results
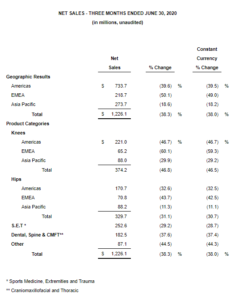
WARSAW, Ind., Aug. 4, 2020 /PRNewswire/ — Zimmer Biomet Holdings, Inc. (NYSE and SIX: ZBH) today reported financial results for the quarter ended June 30, 2020. The Company reported second quarter net sales of $1.226 billion, a decrease of 38.3% from the prior year period, and a decrease of 38.0% on a constant currency basis. Net loss for the second quarter was $206.6 million. […]
Xtant Medical Announces Second Quarter 2020 Financial Results
BELGRADE, Mont., Aug. 03, 2020 (GLOBE NEWSWIRE) — Xtant Medical Holdings, Inc. (NYSE American: XTNT), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today reported financial and operating results for the second quarter ended June 30, 2020. Second Quarter 2020 Financial Highlights: Revenue for the second quarter of […]
OrthoPediatrics Corp. Achieves 500th Case with FIREFLY® Pedicle Screw Navigation Guides
WARSAW, Ind., Aug. 03, 2020 (GLOBE NEWSWIRE) — OrthoPediatrics Corp. (“OrthoPediatrics” or the “Company”) (Nasdaq:KIDS), a company focused exclusively on advancing the field of pediatric orthopedics, announced the 500th case performed with the FIREFLY® Pedicle Screw Navigation Guides (“FIREFLY”) technology in collaboration with Mighty Oak Medical. FIREFLY, which is frequently used in conjunction with OrthoPediatrics’ RESPONSE™ Spine System, […]
Medicrea announces the appointment of an independent expert
Lyon and New York, August 3, 2020 – The MEDICREA® Group (Euronext Growth Paris: FR0004178572 – ALMED ; OTCQX Best Market -MRNTF), pioneering the transformation of spinal surgery through Artificial Intelligence, predictive modeling and patient specific implants with its UNiD™ ASI (Adaptive Spine Intelligence) proprietary software platform, services and technologies, announces the appointment of an independent […]
8 Anterior Lumbar Plate Systems to Know…!
1.-Binary Lumbar| Genesys Spine The Genesys Spine Binary® Lumbar Plate System provides an innovative solution to address instability of the spine from T1 to S1 caused by fractures, tumors, DDD, scoliosis, kyphosis, lordosis, spinal stenosis, or a failed previous spine surgery. Consists of Titanium plates with integrated locks and Titanium screws. One-Level (Flat) Plates, Intradiscal […]