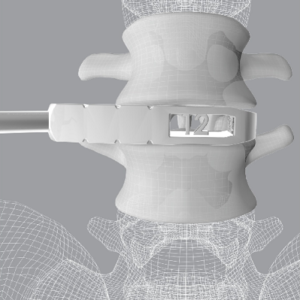
Clariance has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its Stand-Alone Anterior Lumbar Interbody Fusion Device: Idys®-ALIF ZP 3DTi. The design allows four fixation screws to be inserted through the cage and into the adjacent vertebral bodies to create a zero-profile stand-alone construct that removes the need for posterior […]
2020
Implanet: 2020 half-year results
Bordeaux, Boston, September 15, 2020 – 5.45 pm CEST: IMPLANET (Euronext Growth: ALIMP, FR0013470168, eligible for PEA-PME equity savings plans), a medical technology company specializing in vertebral and knee-surgery implants, announces its results for the first half of the year to June 30, 2020, as approved by the Board of Directors on September 15, 2020. Implanet […]
CoreLink Releases First-Of-Its-Kind Oblique Lumbar Interbody Fusion Instrument Set With 3D Printed Instruments
ST. LOUIS, Sept. 15, 2020 /PRNewswire/ — CoreLink, LLC, a leading designer and manufacturer of spinal implant systems, today announced the limited commercial release of the Oblique Lumbar Interbody Fusion (OLIF) Instrument Set. The OLIF technique is a less invasive approach to the lower lumbar spine, which can allow for faster recovery time and minimized psoas disruption. “The addition […]
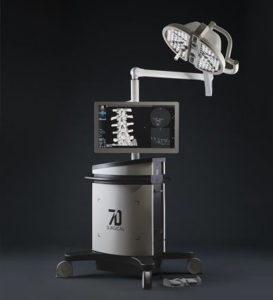
7D Surgical Completes Largest Financing Round to Date
TORONTO, Sept. 15, 2020 /PRNewswire/ — 7D Surgical, a Toronto based medical device company developing advanced optical and machine-vision technologies for surgical navigation, announced today the completion of its recent financing round. This investment follows its successful commercialization in the United States and its recent launch into Southeast Asia. The financing will provide 7D with the capital to grow existing operations, further develop […]
Spine Align Raises $1.75M Series Seed Round to Reach FDA Clearance
BALTIMORE, Sept. 14, 2020 /PRNewswire/ — Spine Align, LLC, a medical device company developing a portfolio of products that enable spine surgeons to measure, visualize, and adjust patients’ spinal alignment during surgery, recently closed a $1.75M Series Seed round. The financing is led by the Rockies Venture Club, with additional support by members of the Berkeley Angel Network, NO/LA Angel […]
CTL Amedica Sets Sights on Asian Market: Receives TFDA License for Three Products
DALLAS (PRWEB) SEPTEMBER 10, 2020–The Taiwan Food and Drug Administration (TFDA) has granted CTL Amedica Corporation a license for three silicon nitride spine products – the Valeo II Lumbar Interbody Fusion Device, number 033665; the Valeo C+CSC Cervical Interbody Fusion Device, number 033666; and the Valeo II Cervical Interbody Fusion Device, number 033667. The official license […]
IZI Medical Receives CE Mark Approval for Kiva™ VCF Treatment System
OWINGS MILLS, Md., Sept. 10, 2020 /PRNewswire/ — IZI Medical Products, LLC (“IZI”), a leading manufacturer of interventional radiology devices, announces that it has received CE Mark approval in Europe for the KivaTM Vertebral Compression Fracture (VCF) Treatment System. Kiva is a unipedicular PEEK implant-based treatment solution for VCFs that has seen clinical and commercial success in the US. “We […]
Spineway: Launch of MISTI, a modular platform for instruments
Spineway, specialized in the treatment of severe disorders of the spine, announces the launch of a new concept: MISTI (acronym for Minimally Invasive Spine Total Instrumentation).It is a new modular platform for instruments used in the treatment of spinal disorders based on the Mont Blanc MIS pedicle screw, which allows surgeons to choose, mix and […]
DiscGenics Appoints Former Medtronic Spine and Biologics Finance Executive as First Chief Financial Officer
SALT LAKE CITY, Sept. 9, 2020 /PRNewswire/ –DiscGenics, Inc., a clinical stage biopharmaceutical company developing regenerative cell-based therapies that alleviate pain and restore function in patients with degenerative diseases of the spine, today announced it has appointed former Medtronic Spine and Biologics finance executive, Jeff Poole, as its first Chief Financial Officer (CFO). Mr. Poole has over 13 […]
Neil P. Dougherty Appointment Vice President of Sales for Spinal Simplicity’s Spine Division
OVERLAND PARK, KAN. (PRWEB) SEPTEMBER 06, 2020–Spinal Simplicity, a medical device company, today announced that Neil P. Dougherty has been appointed Vice President of Sales for its Spine Division. “We are delighted to welcome Neil to our team,” said Todd Moseley, CEO, and Co-Founder of Spinal Simplicity. “We were looking for a high character, high performing […]
MEDICREA Reports First Half 2020 Results
Lyon and New York, September 4, 2020 – The MEDICREA® Group (Euronext Growth Paris: FR0004178572– ALMED ; OTCQX Best Market –MRNTF), pioneering the transformation of spinal surgery through Artificial Intelligence, predictive modeling and patient specific implants with its UNiD™ ASI (Adaptive Spine Intelligence) proprietary software platform, services and technologies, reports its unaudited results for the […]
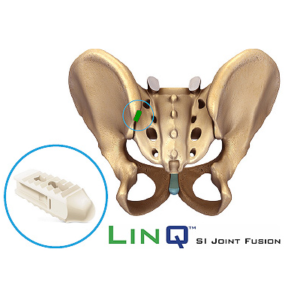
Expert Pain Care to Offer New Innovative LinQ(TM) Procedure
HOUSTON, Sept. 4, 2020 /PRNewswire/ — Dr. Ioannis Skaribas, founder of Expert Pain Care in Houston, Texas, is excited to announce that they will provide a new, minimally invasive treatment of sacroiliac (SI) joint pain by PainTEQ. For patients with SI joint pain and previous treatments that have been unsuccessful, this new procedure, the LinQ™ Sacroiliac Joint Fusion […]
More appealing, more informative and with new functions: the revised SIGNUS Medizintechnik website is online!
Alzenau, August 2020–Since the end of August SIGNUS presents itself with a new website! The new online presence offers clearly arranged and comprehensive information about the family business SIGNUS, its products and events. Doctors, healthcare professionals and distributors will find optimally adjustedcontent that is presented in an up-to-date and clear layout.For patients, there is a […]
FDA grants CTL Amedica 510k clearance for MONDRIAN LIF Cage System
DALLAS (PRWEB) SEPTEMBER 02, 2020 CTL Amedica Corporation is proud to announce that it has received official 510k clearance from the U.S. Food and Drug Administration (FDA) to market its MONDRIAN™ Lumbar Interbody Fusion (LIF) Cage System featuring TiCro™ surface technology, a unique and innovative approach to titanium interbody fusion devices. “Using our CEZANNE™ LIF System […]
Wishbone Medical Names Jodie N. Heggelund Global President of Spine & Biologics
WARSAW, Ind., Sept. 02, 2020 (GLOBE NEWSWIRE) — WishBone Medical, Inc., a leader in pediatric orthopedic medical devices, announced this morning that Jodie N. Heggelund will serve as the company’s Global President of Spine & Biologics, effective immediately. Building on recent acquisitions, WishBone is positioned to transform pediatric spine surgery with a platform of anatomically appropriate […]
Royal Biologics Announces the Acquisition of FIBRINET
HACKENSACK, N.J., Sept. 1, 2020 /PRNewswire/ — Royal Biologics, an ortho-biologics company specializing in the research and advancement of autologous and live cellular solutions, today announced the completed acquisition of FIBRINET, from Vertical Spine LLC. The acquisition comes as part of Royal Biologics’ strategic initiative to add novel technologies to its growing portfolio of autologous and live cellular […]
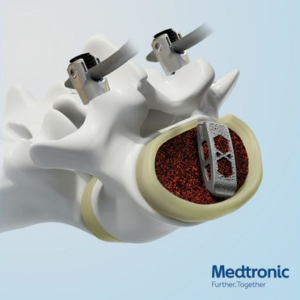
Medtronic Launches ADAPTIX Interbody Cage in Europe
Medtronic launches in Europe the ADAPTIX Interbody system. It is the first fully navigated TLIF device to feature the Titan NanoLOCK Surface Technology. The Adaptix™ Interbody System with Titan nanoLOCK™ Surface Technology consists of additive manufactured titanium cages of various lengths and heights to accommodate patient anatomy. The interbody device has been treated with Titan […]
Hyprevention is launching V-STRUT in the U.S
Hyprevention has started in the US to introduce their new product V-STRUT with Dawa Medical LLC. March 2020, Hyprevention received FDA 510(k) clearance for the V-STRUT© Vertebral Implant, indicated for the treatment of vertebral fractures. V-STRUT© Vertebral implant is an unique new product indicated to treat vertebral fractures using pedicle anchorage and material close to […]
SeaSpine Announces Full Commercial Launch of the Shoreline RT® Cervical Interbody Implant System
CARLSBAD, Calif., Aug. 27, 2020 (GLOBE NEWSWIRE) — SeaSpine Holdings Corporation (NASDAQ: SPNE), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today announced the full commercial launch of the Shoreline RT Cervical Interbody Implant System. Shoreline RT describes a series of products that enhance NanoMetalene® technology with the Company’s […]
Wenzel Spine Announces Acquisition of Statera Spine
AUSTIN, TX August 27, 2020 – Wenzel Spine, Inc., a medical technology company focused on providing minimally invasive solutions for the treatment of spinal disorders, today announced that it has completed the acquisition of Statera Spine, a pre-operative diagnostics and quantitative data software solution that improves treatment decisions for spine patients. Statera Spine is a […]
1st JASPIS ST® Surgery in Australia
Australia, August 2020–Neurosurgeon Dr. Andrew Miles (FRACS MBBS), Neurospine Institute, Western Australia successfully completed the first JASPIS® ST surgery in Australia. Congratulations! Dr. Miles’ first JASPIS® ST case was a patient with degenerative disc disease and cervical myeloradiculopathy. Operative levels were performed at C5/6 & C6/7. The implants successfully restored height and decreased the compression […]
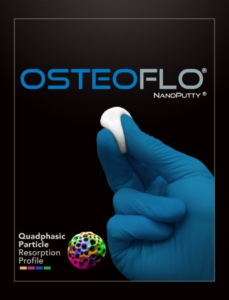
SurGenTec® Announces FDA Clearance for OsteoFlo® NanoPutty® (Quadphasic Synthetic Bone Graft)
SurGenTec, a privately held spine and orthopedic technology company, announced today that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its proprietary OsteoFloNanoPutty-Quadphasic Synthetic Bone Graft. OsteoFloNanoPutty is a novel bone graft solution featuring the world’s first, and only quadphasic synthetic bone graft particles with nano-surface technology. OsteoFloNanoPutty has […]
IZI Medical Products Announces the Launch of its Osteo-Site Vertebral Balloon and Its Complete VCF Treatment Toolbox
OWINGS MILLS, Md., Aug. 25, 2020 /PRNewswire/ — IZI Medical Products, LLC (“IZI”), a leading manufacturer of interventional radiology devices, announces the official launch of its Osteo-Site Vertebral Balloon (“Osteo-Site Balloon”) for vertebral augmentation. The Osteo-Site Balloon completes IZI’s offering of Vertebral Compression Fracture (“VCF”) treatment options, which include Osteo-Site Vertebroplasty, Osteo-Site Balloon Kyphoplasty, Blazer Curved Needle […]
Zavation Medical Products, LLC., Launches Titanium/PEEK Posterior LEIF Cage
JACKSON, Miss., Aug. 25, 2020 /PRNewswire/ — Zavation Medical Products (“Zavation” or the “Company”), an innovative designer and manufacturer of high-quality spinal implants, instruments, MIS procedural kits, and biologics headquartered in Flowood, MS, announced the launch of the Titanium/PEEK Posterior LEIF (Lateral Expandable Interbody Fusion) cage, a cage designed for use in the lumbar spine as a spinal fusion […]
From our opinion, Which are the three best Expandable PLIF/TLIF systems on the market?
In recent years the market for lumbar expandable interbody implants has been growing. It advantages are many for Minimally Invasive approaches but also trying to maximize Lumbar Lordosis and to control Sagittal and Coronal Alignment. The market for expandable cages is approximately $400 million. Globus Medical is the market leader, followed by many other companies such as Stryker, Nuvasive, LifeSpine, Orthofix, Astura Medical, […]
Spine Implants Global Market Report 2020-30: Covid 19 Growth and Change
New York, July 30, Jul 30, 2020 (GLOBE NEWSWIRE via COMTEX) — New York, July 30, 2020 (GLOBE NEWSWIRE) — Reportlinker.com announces the release of the report “Spine Implants Global Market Report 2020-30: Covid 19 Growth and Change” – https://www.reportlinker.com The global spine implants market is expected to decline from $12.44 billion in 2019 to $11.83 […]
Medtronic Reports First Quarter Financial Results
DUBLIN, Aug. 25, 2020 (GLOBE NEWSWIRE) — Medtronic plc (NYSE:MDT) today announced financial results for its first quarter of fiscal year 2021, which ended July 31, 2020. The company reported first quarter worldwide revenue of $6.507 billion, a decrease of 13 percent as reported. After adjusting for the $104 million negative impact of foreign currency translation, the […]