BETHLEHEM, Pa., Oct. 6, 2020 /PRNewswire/ –Tyber Medical, LLC, a leading manufacturer of private label orthopedic and spinal implants, providing rapid access to FDA-cleared and CE-marked devices in the spine, extremity and trauma markets, has launched a clinical study focusing on its titanium-integrated interbody fusion devices for use in the cervical and lumbar spine. The study aims to […]
2020
SeaSpine Provides Preliminary Results for Third Quarter 2020 and Revenue Guidance for Fourth Quarter 2020
CARLSBAD, Calif., Oct. 06, 2020 (GLOBE NEWSWIRE) — SeaSpine Holdings Corporation (NASDAQ: SPNE), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, announced today preliminary financial results for the third quarter of 2020 and provided a revenue outlook for the fourth quarter of 2020. The Company announced preliminary third […]
ATEC Announces Novel PTP Approach
CARLSBAD, Calif., Oct. 05, 2020 (GLOBE NEWSWIRE) — Alphatec Holdings, Inc. (“ATEC” or the “Company”) (Nasdaq: ATEC), a medical device company dedicated to revolutionizing the approach to spine surgery, announced today that over 500 prone transpsoas, or PTP™, surgeries have been successfully performed at multiple sites across the United States. The Company is on track to […]
Aurora Spine’s Patent Related to DEXA Technology™ Patient-Matched Implant Technology Issued by the United States Patent Office
CARLSBAD, Calif., Oct. 05, 2020 (GLOBE NEWSWIRE) — Aurora Spine Corporation (“Aurora Spine” or the “Company”) (TSXV: ASG), a designer and manufacturer of innovative medical devices that improve spinal surgery outcomes, today announced the issuance of its United States Patent No: 10,779,954 entitled “Body Density Scan Result-Matched Orthopedic Implants and Methods of Use” for The […]
Spineology Duo™ System Demonstrates Several Benefits and Improved Clinical Outcomes Compared to Traditional Lateral Surgery
Spineology has completed enrollment in the RaDical post-market study of the novel, expandable Duo™ lateral implant. Data from the first 115 patients enrolled demonstrated that the Duo system may offer several benefits to the patient, surgeon, and hospital compared to traditional lateral interbody fusion surgery, including: Reduced neurological deficits following surgery* 33% decrease in total […]
Seaspine Receives CE Mark Approval for Mariner® MIS posterior fixation system
SeaSpine® Mariner® MIS posterior fixation system is now CE Marked! This premier pedicle screw system is built upon the Mariner modular platform and is designed to provide surgeons with Strength in Versatility. The Mariner® MIS Posterior Fixation System is a premier pedicle screw system built upon the Mariner modular platform. Featuring modular threaded technology with thoughtfully designed […]
NuVasive MaXcess® Retractor Featured in Study Further Validating Prone, Single-Position XLIF® Published in The Journal of Neurosurgery: Spine
SAN DIEGO, Oct. 5, 2020 /PRNewswire/ –NuVasive, Inc. (NASDAQ: NUVA), the leader in spine technology innovation, focused on transforming spine surgery with minimally disruptive, procedurally integrated solutions, today announced the results of the study, “Single-position prone lateral approach: cadaveric feasibility study and early clinical experience,” in The Journal of Neurosurgery: Spine, which features NuVasive’s MaXcess® retractor and further validates prone, […]
SINTX Technologies Appoints Michael Marcroft as Vice President of Business Development
SALT LAKE CITY, Oct. 05, 2020 (GLOBE NEWSWIRE) — SINTX Technologies, Inc. (www.SINTX.com) (NASDAQ: SINT), an original equipment manufacturer (OEM) ceramics company focused on silicon nitride and its applications, announced today that Michael Marcroft has been appointed Vice President of Business Development. In this role, Mr. Marcroft will lead business development initiatives within the medical […]
MCRA CRO Assists Simplify Medical, Inc. on the Fastest Spine PMA Ever Approved by the FDA
WASHINGTON, Oct. 1, 2020 /PRNewswire/ — MCRA, LLC, a leading medical device and biologics advisory firm and Clinical Research Organization (CRO) integrating U.S. and International Regulatory, Clinical Research, Reimbursement, Healthcare Compliance, and Quality Assurance, is pleased to announce its role in the successful management and execution of Simplify Medical, Inc.’s clinical study and approval by the U.S. Food […]
Xtant Medical Announces Closing of Debt Restructuring
BELGRADE, Mont., Oct. 01, 2020 (GLOBE NEWSWIRE) — Xtant Medical Holdings, Inc. (NYSE American: XTNT), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today announced that it has completed its previously announced debt restructuring transaction. The primary purpose of the restructuring was to improve Xtant’s capital structure by […]
SI-BONE, Inc. to Host Virtual KOL Surgeon Panel at NASS
SANTA CLARA, Calif., Oct. 01, 2020 (GLOBE NEWSWIRE) — SI-BONE, Inc., (Nasdaq: SIBN), a Silicon Valley-based medical device company dedicated to solving musculoskeletal disorders of the sacropelvic anatomy, announced today that Jeffrey Dunn, Chairman and CEO, will host a virtual surgeon panel from 10am to 11am ET on October 8, 2020. The panel will include […]
Orthofix FIREBIRD SI Fusion System Receives Additional FDA Clearance for Nanotechnology
LEWISVILLE, Texas–(BUSINESS WIRE)–Oct. 1, 2020– Orthofix Medical Inc. (NASDAQ:OFIX), a global medical device and biologics company with a spine and extremities focus, today announced the U.S. Food and Drug Administration (FDA) 510(k) clearance for the nanotechnology feature of the FIREBIRD™ SI Fusion System. Introduced earlier this year, the FIREBIRD SI Fusion System is the first 3D-printed titanium bone screw with […]
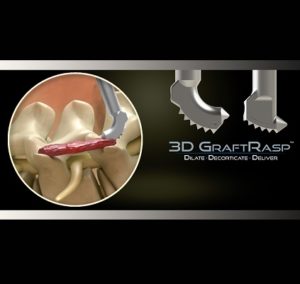
SurGenTec® Announces First-of-its-Kind FDA Clearance for its 3D GraftRasp System™ Including Key Spine Indications
BOCA RATON, Fla., October 1, 2020–SurGenTec, a privately held spine and orthopedic technology company dedicated to creating safe and intuitive solutions for surgeons and patients, announced today that it received U.S. Food and Drug Administration (FDA) 510(k) clearance for its 3D GraftRasp System, as well as key spine indications. The 3D GraftRasp System is the […]
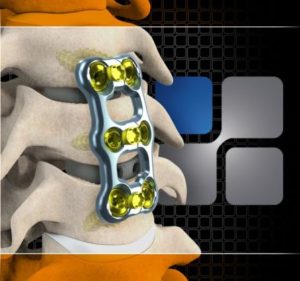
NeuroStructures Launches Transept™ Cervical Plating System
Transept™ Cervical Plating System was designed based upon an emerging trend in the peer reviewed literature identifying a significant decrease in moderate to severe Adjacent Level Ossification when the plate to disc distance is greater than 5mm from the adjacent level. The plate has an ultra-thin profile and comes with a wide spectrum of fixed, […]
Captiva Spine Granted New Patent in the U.S. for Unique Approach to Site Preparation for Posterior SI Joint Fusion Surgery
Captiva Spine, Inc., developer and distributor of intuitive spine fusion devices, announced today they received a “Notice of Allowance” by the United States Patent and Trademark Office (USPTO) for their patent on a novel posterior sacroiliac (SI) joint fusion System named TransFasten. Specifically, the system’s precision form of Quadracentric™ carpentry which prepares the sacroiliac joint intended for an easy, safe, and repeatable SI joint fusion surgery. “Recognizing the growing adoption of SI fusion procedures, we are committed to the continued development of differentiated and proprietary devices that clearly provide incremental improvement. This patent […]
Surgalign Holdings, Inc. Announces Agreement to Acquire Holo Surgical Inc. and its ARAI™ Digital Surgery Platform
DEERFIELD, Ill., Sept. 29, 2020 (GLOBE NEWSWIRE) — Surgalign Holdings, Inc. (Nasdaq: SRGA), a global pure-play spine company focused on advancing spine surgery including through the application of digital technologies to improve patient outcomes, today announces that it has entered into a definitive agreement to acquire Holo Surgical Inc., (‘Holosurgical’), a Chicago-based private technology company […]
John Eekhof hired as Director of Marketing. Nexxt Spine announces leadership appointment to support continued growth
September 28, 2020- Noblesville, IN-Mr. Eekhof has joined Nexxt Spine as Director of Marketing with responsibility for market strategy, product management, strategic communications and immediately driving world class product launches stemming from several recent FDA 510 (k) clearances with many more in the pipeline. In this new role, Mr. Eekhof will also continue to help […]
Hyprevention Raises New Financing to Commercialize Spinal Implant Product V-STRUT© in the USA.
PESSAC, France–(BUSINESS WIRE)–Hyprevention has raised new financing to support the V-STRUT© Vertebral Implant product. The financing will be used to execute the commercialization plans for the US spinal fracture market. The V-STRUT© Vertebral Implant is indicated for use in the treatment of vertebral fractures. In May 2019, a US patent was granted for V-STRUT© and […]
Spine Product Launches in 2020? At least 20 New Products…
In 2020, at least 20 spine products have been launched on the market. Due to COVID 19, from the month of March to April, there were no releases. The companies that have been most active in incorporating new systems have been Seaspine (6) and Zavation Medical (3). Both companies have joined the expandable technology. Almost a third of the launches have […]
Spine Wave Announces the Commercial Launch of Tornado® Bioactive Bone Graft Matrix
SHELTON, Conn., Sept. 24, 2020 (GLOBE NEWSWIRE) — Spine Wave is pleased to announce the commercial launch of Tornado® Bioactive Bone Graft Matrix, effective immediately. Tornado® Bioactive Bone Graft Matrix strengthens the company’s growing spinal biologics portfolio and marks Spine Wave’s entry into the market for synthetic bioactive bone graft matrix implants. Tornado® Bioactive Bone Graft Matrix complements […]
SeaSpine® Announces Limited Commercial Launch of WaveForm C 3D-Printed Interbody Implant System
CARLSBAD, CA (September 23, 2020) – SeaSpine Holdings Corporation (NASDAQ: SPNE), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today announced the limited commercial launch of its WaveForm C Interbody Implant System. WaveForm C represents the first of five 3D-printed interbody devices that the Company anticipates launching by […]
Orthofix and Neo Medical Announce Partnership to Develop and Market Single-Use Spinal Solutions Focused on Improving Patient Outcomes
LEWISVILLE, Texas–(BUSINESS WIRE)–Sep. 22, 2020– Orthofix Medical Inc. (NASDAQ:OFIX), a global medical device company focused on musculoskeletal healing products, and Neo Medical SA, a privately held Swiss-based Medtech company developing a new generation of products for spinal surgery, today announced a partnership and investment agreement to develop and market innovative outcome-driven procedural solutions. The collaboration will focus […]
Life Spine Announces FDA 510(k) Clearance for SI Joint Revision Offering and Additional Claims for the SImpact® Sacroiliac Joint Fixation System
Huntley, IL, September 22, 2020 –Life Spine, a medical device company that designs, develops, manufactures and markets products for the surgical treatment of spinal disorders, announced today that it has received clearance from the U.S. Food & Drug Administration (FDA) to market a SI revision offering and additional claims for the SImpact Sacroiliac Joint Fixation System. The new […]
Zavation Medical Products, LLC., Earns Spot As An Inc. 5000 Fastest Growing Company
JACKSON, Miss., Sept. 22, 2020 /PRNewswire/ — Zavation Medical Products (“Zavation” or the “Company”), an innovative designer and manufacturer of high-quality spinal implants, instruments, MIS procedural kits, and biologics headquartered in Flowood, MS, has been recognized as one of 2020’s fastest-growing, privately-held companies in the United States by Inc. 5000. “While our management team is honored by the Inc. 5000 recognition, […]
Nexxt Spine Releases Stand Alone Cervical Turn Lock System
September 21, 2020- Noblesville, IN- Nexxt Spine LLC, a leading design and manufacturing company of spinal solutions releases the nexxt generation of their Stand Alone Cervical System with the launch of Stand Alone Cervical Turn Lock (TL). The recently released TL System incorporates the dual functionality of a cervical interbody and anterior plate with the […]
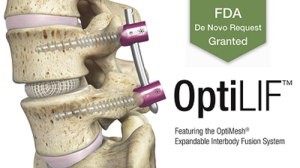
Spineology® Announces FDA De Novo Grant of Minimally Invasive OptiMesh® Expandable Interbody Fusion System
ST. PAUL, Minn.–(BUSINESS WIRE)–Spineology Inc., an innovator in anatomy-conserving surgery, is excited to announce the FDA grant of its proprietary Spineology Interbody Fusion System, now called the OptiMesh Expandable Interbody Fusion System. The grant follows the successful completion of the SCOUT (Spineology Clinical Outcomes Trial) Investigational Device Exemption (IDE) trial. OptiMesh is a unique mesh […]
SPINEMarketGroup YouTube Channel Update: More than 500 Videos added!
Thank you to all the Companies that have shared their videos with us! To date, we have more than 535 VIDEOS on our channel including many of the most relevant products. In the last month, in order to provide you with more information we have created a video channel to host videos about the different […]