SpineGuard (Paris:ALSGD) (FR0011464452 – ALSGD), an innovative company that deploys its DSG® (Dynamic Surgical Guidance) sensing technology to secure and streamline the placement of bone implants, announced today the CE mark of its “DSG Connect” platform. Already being utilized experimentally in a new strategic high value platform to guide surgical robots, SpineGuard will now apply this platform […]
2020
Nexxt Spine Rolls Out Corpectomy System to Surgeons
March 31, 2020- Noblesville, IN- Nexxt Spine LLC, a pioneer in the design and manufacturing of innovative spinal solutions, is pleased to announce the FDA 510(k) clearance of the Nexxt Matrixx® Corpectomy System. Celebrated as the industry leader in 3D printed porous titanium technology, Nexxt Spine is proud to offer this newest addition to their […]
Safe Orthopaedics announces the regulatory approval of SteriSpineTM PS 2nd Generation in Japan
Éragny-sur-Oise, France, March 31st 2020 – Safe Orthopaedics (FR0013467123 – ALSAF), a company specialized in the design and marketing of ready-to-use technologies for spinal surgery, delivering the safest treatment of spinal fractures urgently treated, announces the regulatory approval of SteriSpine™ PS 2nd Generation in Japan. In July 2019, Safe Orthopaedics had announced the commercial launch of SteriSpine™ PS […]
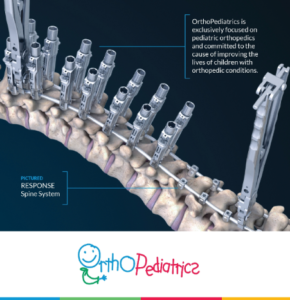
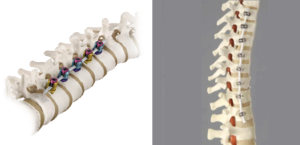
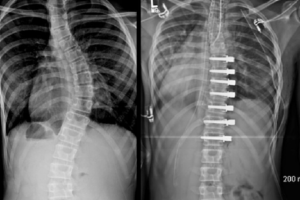
OrthoPediatrics Corp. Receives FDA 510(k) Clearance and Expanded Neuromuscular Indications for its RESPONSE™ Scoliosis System
WARSAW, Ind., March 31, 2020 (GLOBE NEWSWIRE) — OrthoPediatrics Corp. (“OrthoPediatrics”) (Nasdaq: KIDS), a company focused exclusively on advancing the field of pediatric orthopedics, today announced it has received 510(k) clearance from the U.S. Food and Drug Administration to expand the indications for its RESPONSE™ Scoliosis System to include neuromuscular implants. This 510(k) clearance represents a significant […]
2 Thetering Systems to know…!
Vertebral Body Tethering is a non-fusion spinal procedure intended to treat idiopathic scoliosis, an abnormal curvature in the spine that occurs without a known cause, in young patients whose bones have not fully matured. The Vertebral Body Tethering System are made up of: anchors, bone screws, cord, and set screws. The anchors, bone screws and set […]
Rope or rod? Torn between scoliosis surgeries
For a long time, spinal fusion was the only surgery to treat scoliosis. Over the years, the operation has improved and become the gold standard. But now, there’s another procedure available. Though it seems to offer big advantages, its long-term outcomes are uncertain. How do you weigh one against the other? We recommend you this […]
Globus Medical Announced $200 Million Share Repurchase Program
AUDUBON, Pa., March 11, 2020 (GLOBE NEWSWIRE) — Globus Medical, Inc. (NYSE:GMED), a leading musculoskeletal solutions company, announced the Board of Directors authorized the repurchase of $200 million of the Company’s common stock. “We believe the recent stock market volatility has created a significant divergence between the intrinsic value of Globus Medical and its value […]
MEDICREA Is Protected From the Consequences of the COVID-19 Pandemic
The MEDICREA® Group (Euronext Growth Paris: FR0004178572 – ALMED ; OTCQX Best Market –MRNTF), pioneering the transformation of spinal surgery through Artificial Intelligence, predictive modeling and patient specific implants with its UNiD™ ASI (Adaptive Spine Intelligence) proprietary software platform, services and technologies, communicates on the consequences on its activity of the COVID-19 pandemic and the […]
Safe Orthopaedics: the European Patent Office Confirms the Validity of Several Key Patents
Safe Orthopaedics (Paris:ALSAF) (FR0013467123 – ALSAF), a company marketing innovative ready-to-use technologies (single-use implants and instruments) for spinal disease, delivering the safest treatment of spinal fractures, summarizes its strategy’s progress on industrial property. To this day, the company holds a portfolio of nearly 100 delivered patents, covering mainly Europe, United States, China, Canada and Japan. […]
SINTX Technologies Files Patent Related to Antipathogenic Compositions and Methods
SALT LAKE CITY, UT, March 23, 2020 (GLOBE NEWSWIRE) — SINTX Technologies, Inc. (NASDAQ: SINT) (“SINTX” or the “Company”), an original equipment manufacturer (OEM) ceramics company focused on silicon nitride applications, today announced that US and international patent applications were published on March 12, 2020 in which certain claims made in the applications address the […]
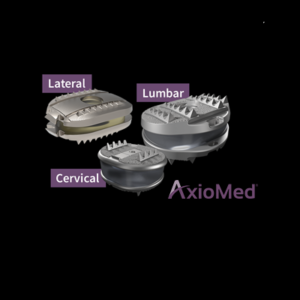
KICVentures Appoints Ken Yamada as President & CEO of AxioMed
BOSTON (PRWEB) MARCH 18, 2020–AxioMed, LLC is dedicated to advance the standard of care for patients with degenerative spine conditions by progressing spine technology beyond fusion and first generation artificial discs and has announced Ken Yamada as its President and Chief Executive Officer succeeding Kingsley Chin, MD., who is stepping down to focus on further expanding […]
KICVentures Appoints Jake Lubinski as CEO of NanoFUSE Biologics
BOSTON (PRWEB) MARCH 19, 2020 NanoFUSE Biologics, LLC, the only biologics company with a bioactive glass FDA-cleared to be combined with DBM, announced it has chosen Jake Lubinski, the current President of NanoFUSE Biologics, LLC and President of Commercialization and Acquisition of parent company KICVentures, LLC, as its new CEO effective March 16th 2019. In January […]
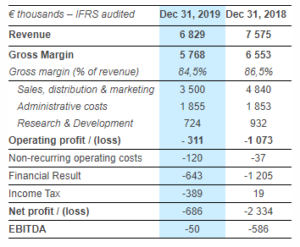
SpineGuard Reports Solid Improvements in Its Full-year 2019 Financial Results
SpineGuard (Paris:ALSGD) (FR0011464452 – ALSGD), an innovative company that deploys its DSG® (Dynamic Surgical Guidance) sensing technology to secure and streamline the placement of bone implants, reported today its full-year 2019 financial results as approved by the Board of Directors on March 19, 2020. Pierre Jérôme, co-founder, Chairman and CEO of SpineGuard, said: “The reorganization plan we […]
4 Companies that changed CEO Recently!
1.-CENTINEL SPINE Centinel Spine®, LLC, announced Steven F. Murray as CEO effective March 16, 2020, succeeding the current Chairman & CEO John J. Viscogliosi, who was stepping down to focus his efforts on further expanding Viscogliosi Brothers, LLC’s 20 years of leadership in the development of innovative companies in the neuromusculoskeletal segment of healthcare. Steven […]
Centinel Spine Announces Steven F. Murray as CEO
New York, NY, March 16, 2020 – Centinel Spine®, LLC, the largest privately-held spine company focused on anterior column reconstruction, today announced Steven F. Murray as CEO effective March 16, 2020, succeeding the current Chairman & CEO John J. Viscogliosi, who is stepping down effective immediately to focus his efforts on further expanding Viscogliosi Brothers, LLC’s […]
11 Cervical Artificial Disc to Know..!
Global artificial disc market is predicted to attain revenue of $3.3 billion by 2024, according to a market research report published by P&S Intelligence.Cervical discs are expected to generate revenue of $2,479.6 million by 2024. According to iData Research, Inc. , the Cervical Artificial Disc Segment is expected to Comprise Nearly €57 million of the […]
Thanks to the Doctors and Nurses that are on the front line against COVID-19!
As the world faces a global pandemic, there are millions of people we all should be thanking—the doctors, nurses, and other medical personnel who are on the front line of the war against COVID-19. Also, I would like to give recognition to all the Medical Devices companies with all their distributors, sales people, and product […]
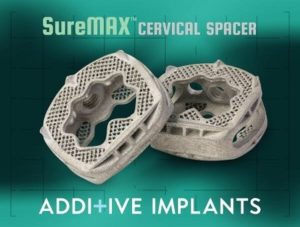
Additive Implants Achieves Milestone of 200 Levels Treated
PHOENIX, March 12, 2020 /PRNewswire/ — Additive Implants announced today that the company has reached the milestone of 200 spinal levels treated with the SureMAX™ Cervical Spacer. The milestone procedure was performed by Dr. Rafath Baig at Banner Desert Medical Center in Phoenix, Arizona. Dr. Baig commented: “I have been very pleased with the performance of the spacer and its unique surface […]
‘Tiger Woods Surgery’ available in Rome
The Tiger Woods minimally invasive spine surgery using Centinel Spine’s STALIF M-Ti™ fusion implant that allowed Woods to complete his PGA Tour comeback after almost three years of golf inactivity, is now available at Rome Memorial Hospital. The Director of Neurosciences at Rome Memorial Hospital, Dr. Nicholas Qandah, a board certified neurosurgeon and Board Certified General Surgeon Dr. Keneth […]
Randomized Controlled Trial Results Published in ‘Spine’ Demonstrate Efficacy of NuVasive Attrax® Putty in Posterolateral Lumbar Fusion
SAN DIEGO, March 10, 2020 /PRNewswire/ — NuVasive, Inc. (NASDAQ: NUVA), the leader in spine technology innovation, focused on transforming spine surgery with minimally disruptive, procedurally integrated solutions, today announced the results of the study “Efficacy of a Standalone Microporous Ceramic vs. Autograft in Instrumented Posterolateral Spinal Fusion; a Multicenter, Randomized, Intra-patient Controlled, Non-inferiority Trial” published online in Spine. The […]
10 MIS Extended Tabs Screws systems that are Worth to Know…!
The minimally invasive spine (MIS) surgery market, will rise in 2020 to $900 million worldwide. It accounts for approximately 10% of the Global Spine. Minimally Invasive spinal surgeries are growing in popularity due to its advanced features, such as high accuracy, less blood loss, reduced hospital stay, and favorable reimbursement policies. Traditional open surgeries can cause various post-operation […]
ATEC Reports Fourth Quarter and Full Year 2019 Financial Results
CARLSBAD, Calif., March 05, 2020 (GLOBE NEWSWIRE) — Alphatec Holdings, Inc. (“ATEC” or the “Company”) (Nasdaq: ATEC), today announced financial results for the fourth quarter and full year ended December 31, 2019, and recent corporate highlights. Fourth Quarter and Full Year 2019 Financial Highlights 2019 and Recent Commercial and Product Highlights Increased the percent of […]
Xtant Medical Announces Fourth Quarter and Full Year 2019 Financial Results
BELGRADE, Mont., March 05, 2020 (GLOBE NEWSWIRE) — Xtant Medical Holdings, Inc. (NYSE American: XTNT), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today reported financial and operating results for the fourth quarter and year ended December 31, 2019. Fourth Quarter 2019 Financial Highlights and Recent Announcements: Revenue […]
SINTX Technologies Releases Preliminary 2019 Year End Earnings Report and Business Update
SALT LAKE CITY, UT, March 04, 2020 (GLOBE NEWSWIRE) — SINTX Technologies, Inc. (NASDAQ: SINT) (the “Company”), an original equipment manufacturer (OEM) ceramics company that develops and commercializes silicon nitride, today announced preliminary financial results for the year ended December 31, 2019 and provided a business update. YEAR END 2019 FINANCIAL RESULTS SINTX reported revenue […]
Amplify™ Surgical Inc. Reaches Milestone of 300 Levels Treated with dualX® Expanding Interbody Fusion System
Amplify Surgical, Inc., today announces that the company reached the milestone of treating 300 spinal levels with the dualX Expanding Interbody Fusion System. The landmark case was performed by Dr. Ali Najafi at Fresno Heart and Surgical Hospital in Fresno, CA. After the case Dr. Najafi noted, “dualX has completely changed the game for expandable […]
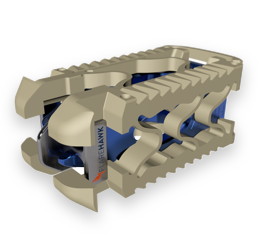
Clinical Study Demonstrates Favorable Patient Outcomes with the FlareHawk® Expandable Cage
PALM BEACH GARDENS, Fla., March 03, 2020 (GLOBE NEWSWIRE) — Integrity Implants Inc., a privately held medical device company dedicated to delivering innovative solutions for spine surgery, today announced positive data from a retrospective study demonstrating favorable fusion efficacy with its FlareHawk® interbody implant. The study, “Transforaminal/Posterior Lumbar Interbody Fusion with the FlareHawk Expandable Interbody Fusion Device,” […]
Implanet Steps Up Development in Germany and Consolidates Its Organization
IMPLANET (Paris:ALIMP) (Euronext Growth: ALIMP, FR0013470168, eligible for PEA-PME equity savings plans), a medical technology company specializing in vertebral and knee-surgery implants, is reinforcing its business operations in Germany, a strategic market for the company. Implanet, which operates in Germany via its subsidiary based in Frankfurt (Implanet GmbH), has appointed Stephan Collardey as Country Manager […]