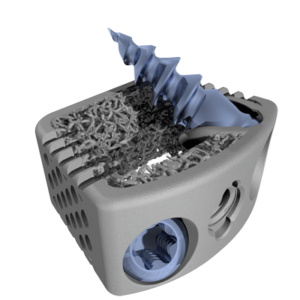
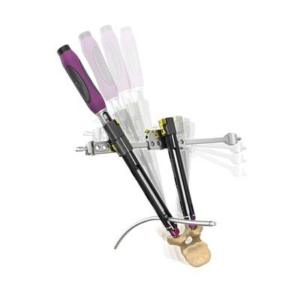
ST. LOUIS–(June 30, 2020)–CoreLink, LLC, a leading designer and manufacturer of spinal implant systems, today announced the commercial launch and 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the F3D-C2 Stand-alone Cervical System. The system is comprised of an additively manufactured spacer with two bone screw anchors secured by a locking mechanism […]
2020
RTI Surgical Holdings, Inc.® Provides Business Updates
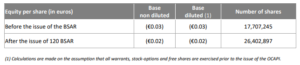
DEERFIELD, Ill., June 30, 2020 (GLOBE NEWSWIRE) — RTI Surgical Holdings, Inc. (Nasdaq: RTIX), a global surgical implant company, today provided a business update in conjunction with releasing financial and operating results for the quarter ended March 31, 2020. Business Update Highlights: Scheduled special meeting of stockholders for July 15, 2020 to vote on sale […]
Stryker extends cash tender offer for all outstanding shares of Wright Medical
Kalamazoo, Michigan, June 29, 2020 (GLOBE NEWSWIRE) — Stryker (NYSE: SYK) announced today that Stryker B.V., an indirect, wholly owned subsidiary of Stryker, has extended the offering period of its previously announced cash tender offer for all outstanding ordinary shares of Wright Medical Group N.V. (NASDAQ: WMGI). The tender offer is being made pursuant to […]
Nexxt Spine Launches Career Page and Array of Job Openings
June 29, 2020- Noblesville, IN- The past few months the team at Nexxt Spine has been full steam ahead designing and manufacturing some of the most recognized spinal solutions on the market. In fact, the quickly emerging name in the industry is on the upswing and looking to add employees across a few of the […]
SpineGuard announces first surgeries with its new “DSG Connect” platform
PARIS and BOULDER (CO), June 29th, 2020 – 08:00am CEST – SpineGuard (FR0011464452 – ALSGD), an innovative company that deploys its DSG® (Dynamic Surgical Guidance) sensing technology to secure and streamline the placement of bone implants, announced today the first cases using its “DSG Connect” platform. They were performed successfully by Prof. Assaker and his […]
6 PLIF/TLIF Cages that you will know soon…
According to Allied Market Research, the global interbody fusion cage market is projected to reach $2,309 million by 2023.The lumbar segment capture the highest share of about 30% and is expected to remain dominant throughout 2023. The main trends in the Lumbar cages market are the following: Lateral and ALIF cages Stand Alone interbody fusion […]
SpineGuard secures €2.4M equity line with Nice & Green
PARIS and BOULDER (CO), June 26, 2020 – 18:00 CEST – SpineGuard (FR0011464452 – ALSGD), an innovative company that deploys its DSG® (Dynamic Surgical Guidance) sensing technology to secure and streamline the placement of bone implants, announces today that it has secured a €2.4M financing in the form of an equity line made of 120 […]
New partnership: joimax® joins forces with JAPAN MEDICALNEXT CO., LTD.
Karlsruhe (GER) – joimax®, the German-based market leader in technologies and training methods for full-endoscopic and minimally invasive spinal surgery, is starting as of now a new partnership in Japan. The distributor JMC- JAPAN MEDICALNEXT CO., LTD. and joimax® are pursuing a long-term sales cooperation that will result in an extensive distribution agreement for the entire […]
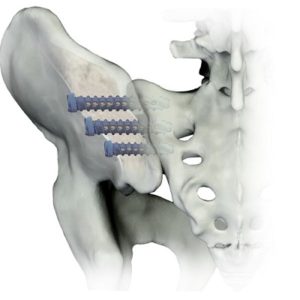
Pantheon Surgical Acquires Blue Topaz/CompresSIve Technology
Pantheon Surgical, a medical technology firm focused on next-generation modular products aimed at sustainability and improved patient outcomes, announces the acquisition of Blue Topaz/CompresSIve sacroiliac screw system. Pantheon Surgical acquired the technology from Osseus Fusion Systems, who will continue to distribute the Blue Topaz/CompresSIve system. Pantheon Surgical is excited for the addition of a front […]
Bioventus Receives FDA Clearance of Strip Format of its SIGNAFUSE® Bioactive Bone Graft
DURHAM, NC – June 25, 2020 – Bioventus, a global leader in orthobiologic solutions, is launching its SIGNAFUSE Bioactive Bone Graft in a new strip format. The strips consist of 55% bioglass by weight and have been shown to induce higher levels of osteoblast differentiation compared to other synthetic bone graft strips.1 SIGNAFUSE has been available as a putty since […]
Mighty Oak Medical has reached a strategic partnership agreement with the Spanish multinational MBA Surgical Empowerment to market the Firefly® surgical guide system in Spain and Portugal
June 2020 –Mighty Oak Medical, an American projects’ incubator and leader in specialised solutions for the spine, has reached a strategic agreement with MBA Surgical Empowerment , a Spanish company dedicated to the distribution of surgical technology, to market the surgical guide system Firefly® in Spain and Portugal. Firefly® is a revolutionary system of 3D […]
SeaSpine® Announces Limited Commercial Launches of NorthStar™ OCT and Cervical Facet Fusion™ Systems
CARLSBAD, CA (June 22, 2020) – SeaSpine Holdings Corporation (NASDAQ: SPNE), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, today announced the limited commercial launches and completion of initial surgeries of both its NorthStar OCT and Cervical Facet Fusion systems, which significantly expand its procedural offerings for posterior […]
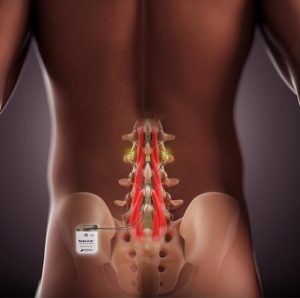
Mainstay Medical Announces U.S. FDA Approval of ReActiv8® Neurostimulation System for Chronic Low Back Pain
Dublin – Ireland, 22 June 2020 – Mainstay Medical Holdings plc (“Mainstay” or the “Company”) today announced that the U.S. Food and Drug Administration (FDA) has approved the Company’s Premarket Approval (PMA) application for ReActiv8®, its implantable neurostimulation system to treat intractable chronic low back pain. Jason Hannon, CEO of Mainstay, said: “I am so proud of […]
7 ALIF Cages that you will know soon…
These seven ALIF cages have already received U.S. Food and Drug Administration (FDA) 510(k) clearance or CE Mark approval . Most of them have started with the first surgeries or they will be launched during the next months. 1.- El Capitan | Astura Medical On April 29, 2020 Astura Medical, received 510(k) clearance from the U.S. […]
NuVasive Expands Complex Spine Portfolio with Global Launch of Reline 3D for Pediatric Deformity
SAN DIEGO, June 18, 2020 /PRNewswire/ — NuVasive, Inc. (NASDAQ: NUVA), the leader in spine technology innovation, focused on transforming spine surgery with minimally disruptive, procedurally integrated solutions, today announced the expansion of its complex spine portfolio with the global commercial availability of Reline 3D™, a posterior fixation system for patients suffering from pediatric spinal deformities. The Reline […]
Aesculap Celebrates Five-Year Anniversary of the FDA Approval of the activL® Artificial Disc: Five Years and Five Milestones
CENTER VALLEY, Pa., June 18, 2020 /PRNewswire/ — Aesculap Implant Systems, LLC today announced that it has been five years since the organization received FDA approval to market the activL® Artificial Disc in the United States. In the last five years, the data behind lumbar arthroplasty along with patients’ access to this procedure has continued to grow. Several significant milestones […]
icotec ag Receives Approvals for Two KONG® VBR Spinal Systems Made with BlackArmor® and Ti-iT® in Europe and the US
ALTSTAETTEN, Switzerland, June 18, 2020 /PRNewswire/ — icotec ag announces that the KONG®-TL and the KONG®-C vertebral body replacement systems with the unique Titaniumcoating (Ti-iT®) receive FDA 510(k) clearance in the United States and CE approval in Europe .”This is exciting news for icotec ag and our team as it allows us to expand our portfolio in multiple countries simultaneously, to include implants […]
Someone has to say it! Thank you Mr Demski!
A few days ago, I found this letter from the CEO of Globus Medical on Linkedin. It seemed to me that finally someone important had something to say and said it. We do not work at Globus Medical, nor for them, but Dave Demski undoubtedly has our respect and admiration. The letter was the following: […]
Spinal Elements® Introduces its MIS Ultra™ Platform of Products and Procedures Designed to Minimize the Unintended Consequences of Spine Surgery
Carlsbad, CA – June 17, 2020 – Spinal Elements, a Carlsbad, CA-based medical device company focused on spine surgery procedures, today introduced its MIS Ultra™ platform of products and procedures. MIS Ultra is a suite of minimally invasive integrated instrument and implantable device solutions designed to optimize spinal fixation and restoration while minimizing the unintended […]
Safe Orthopaedics confirms the acquisition of LCI Medical and holds its General Assembly on July 24th, 2020
Eragny-sur-Oise, France, June 16th, 2020, 17h45 CEST – Safe Orthopaedics (FR0013467123 – ALSAF), a company specializing in the design and marketing of ready-to-use technologies for spinal surgeries, delivering the safest treatment of spinal fractures urgently treated, confirms the acquisition of LCI Medical following the decision of the board of directors on June 12th, 2020, and the […]
Zavation Medical Products, LLC, Launches Cortical Screw
JACKSON, Miss., June 16, 2020 /PRNewswire/ — Zavation Medical Products (“Zavation” or the “Company”), an innovative designer and manufacturer of high-quality spinal implants, instruments, MIS procedural kits, and biologics headquartered in Flowood, MS, announced the launch of the Cortical Screw, a screw designed for use in the cortical bone as an extension of the Zavation Spinal System. The Cortical […]
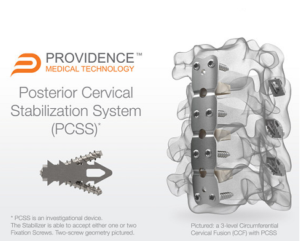
Providence Medical Technology Announces First Patient Enrollment in the “FUSE” IDE Clinical Study
PLEASANTON, Calif., June 15, 2020 /PRNewswire/ — Providence Medical Technology, Inc., a medical device innovator focused on improving surgical outcomes of the cervical spine, announced initiation of enrollment in a human clinical Investigational Device Exemption (IDE) study evaluating the safety and effectiveness of its Posterior Cervical Stabilization System (PCSS) in patients with 3-level degenerative disc disease. The […]
6 Lateral Cages that you will know soon…
According to Allied Market Research, the global interbody fusion cage market is projected to reach $2,309 million by 2023.The lumbar segment capture the highest share of about 30% and is expected to remain dominant throughout 2023. This is attributed to the growing popularity of lumbar spine fusion techniques due to their ability to provide long-term […]
MiRus™ Announces First Clinical Use of the MiRus™ 3DR™ Printed Lumbar Interbody Fusion System
ATLANTA, June 11, 2020 /PRNewswire/ — MiRus is pleased to announce that it received FDA 510(k) clearance for its 3DR™ (Randomized) Printed Lumbar Interbody Fusion System which consists of the CALLISTO 3DR™ PLIF, HYPERION 3DR™ TLIF, CALYPSO 3DR™ LLIF and the ANTARES 3DR™ ALIF. “The HYPERION 3DR TLIF is the first interbody with stiffness equivalent to […]
OrthoPediatrics Corp. Commences Initial U.S. Launch of ApiFix’s FDA-Approved Spinal Deformity Correction System
WARSAW, Ind., June 10, 2020 (GLOBE NEWSWIRE) — OrthoPediatrics Corp. (“OrthoPediatrics” or the “Company”) (Nasdaq:KIDS), a company focused exclusively on advancing the field of pediatric orthopedics, is pleased to announce the initial U.S. launch of the ApiFix Minimally Invasive Deformity Correction (“ApiFix”) system. Additionally, the Company anticipates approximately 20 leading clinical centers in the United States to […]
RTI Surgical Holdings, Inc.® Bolsters Spine Business with Key Senior Leadership Appointments
DEERFIELD, Ill., June 10, 2020 (GLOBE NEWSWIRE) — RTI Surgical Holdings, Inc. (Nasdaq: RTIX), a global surgical implant company, today announced two senior leadership appointments to support its transition to a pure-play global spine company. Scott Durall will join RTI as Chief Commercial Officer, and Bryan Cornwall will join RTI as Executive Vice President, Research […]
4WEB Medical Announces FDA 510(k) Clearance of Stand-Alone Anterior Lumbar Interbody Fusion Device
DALLAS, June 9, 2020 /PRNewswire/ — 4WEB Medical, an orthopedic device company focused on developing innovative implants with an Advanced Structural Design that utilizes its proprietary Truss Implant Technology™, announced today that the company has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its Stand-Alone Anterior Lumbar Interbody Fusion Device (ASTS-SA). The new […]