SANTA ANA, Calif., April, 2016 /PRNewswire/ — An Orange County woman has dismissed her lawsuit against HealthSmart Pacific, former owner of Pacific Hospital of Long Beach, after it was revealed that the spinal implants used in her surgery were not counterfeit, as she alleged in the wake of similar, highly publicized claims by a group of […]
2016
Spine Sales Reps Blues.Stormy Weather
April 17, 2016–The last decade has seen an unprecedent growth in the Spine market. Mainly due to the aging population and the development of improved technologies with new and better implants and instruments. Most of us, we have seen how spinal companies were growing and increasing their sales and profits. At the same time , […]
SpinalCyte Announces Publication in Global Spine Journal
HOUSTON–(BUSINESS WIRE)–SpinalCyte, LLC, a Texas-based tissue engineering technology company focused on regrowth of the spinal disc nucleus using Human Dermal Fibroblasts (HDFs), today announced the publication of its discovery surrounding use of HDFs for regrowth and repair of the intervertebral disc in the April 2016 Edition, Issue 02, Volume 06 of the Global Spine Journal. […]
Aurora Spine’s Patent Related to Its ZIP® ISP Allowed by the United States Patent Office
CARLSBAD, CALIFORNIA–(Marketwired – April 14, 2016) – Aurora Spine Corporation (TSX VENTURE:ASG) announced today the allowance by the United States Patent and Trademark Office of United States Patent Application Serial No. 13/865,155 entitled “Dynamic and Non-Dynamic Interspinous Fusion Implant and Bone Growth Stimulation System”. This patent covers Aurora’s family of ZIP® ISPs, including its ZIP […]
VEXIM: Strong Revenue Growth in Q1 2016
TOULOUSE, France–(BUSINESS WIRE)–Regulatory News: VEXIM (FR0011072602 ‐ ALVXM / PEA-PME) (Paris:ALVXM), a medical device company specializing in the minimally invasive treatment of vertebral fractures, today announced its consolidated sales results for the first quarter 2016. Strong growth in the 1st quarter 2016 (Q1) (€ in thousands) Q1 (as of March 31st) Q1 2016 Q1 2015 Change (%) Sales […]
Medtronic Responds to Star Tribune Article Regarding INFUSE Bone Graft
MINNEAPOLIS – April 10, 2016 – This weekend, an article was published in the Minneapolis Star Tribune that criticized Medtronic’s handling of data collected during a retrospective chart review (RCR) of INFUSE Bone Graft between 2006-2008. The article makes insinuations that are false, and fails to include important information regarding the RCR and Medtronic’s actions. […]
Safe Orthopaedics expands its business to the Asia-Pacific
Eragny-sur-Oise, April 13, 2016 – SAFE ORTHOPAEDICS (Euronext: FR0012452746 – SAFOR), a company developing and marketing an innovative range of sterile implants combined with their single-use surgical instruments, is today announcing that the Australian regulatory authorities have approved its SteriSpine ranges and that it has entered into a distribution agreement with SSJ Health covering Australia […]
Implanet commences trading on OTCQX in the U.S.
Bordeaux, Boston, April 13, 2016: IMPLANET (Euronext: IMPL, FR0010458729, PEA-PME eligible; OTCQX: IMPZY), a medical technology company specializing in vertebral and knee-surgery implants, announces that its sponsored Level 1 American Depositary Receipts (ADRs) will commence trading today in the United States on OTCQX® International (“OTCQX”), under the symbol IMPZY, with each ADR representing 2 ordinary […]
EOS imaging Receives FDA Approval for spineEOS
PARIS–EOS imaging (Paris:EOSI) (Euronext, FR0011191766 – EOSI), the pioneer in 2D/3D orthopedic medical imaging, announced today that the U.S. Food and Drug Administration (FDA) has approved spineEOS, an online 3D planning software for spine surgery based on EOS stereo-radiographic 2D/3D imaging. The FDA approval of spineEOS allows EOS to expand its presence in the large […]
Expanding Orthopedics Announces Dale Binke as Vice President of US Sales
OR AKIVA, Israel, April 12, 2016 /PRNewswire/ — Expanding Orthopedics Inc. (EOI), a privately held medical device company focused on developing and commercializing innovative expandable devices for spine surgery, is excited to announce the recent addition of Dale Binke as Vice President of US Sales. In his role, Dale, an industry veteran, will lead US sales and […]
RTI Surgical Launches the Streamline TL Deformity System
ALACHUA, Fla.–(BUSINESS WIRE)–RTI Surgical® Inc. (RTI) (Nasdaq: RTIX), a leading global surgical implant company, is pleased to announce the launch of the Streamline TL Spinal Fixation System – Deformity Instrumentation for complex adult deformities or curvatures. With a focus on versatility, ease-of-use and surgeon comfort, the Streamline TL deformity instruments bring another level of functionality […]
Stryker Acquires SafeWire Product Portfolio
ALLENDALE, N.J.–(BUSINESS WIRE)–Stryker’s Spine division today announced the acquisition of the SafeWire product portfolio, including the Y-Wire guidewire and Tiger Jamshidi Needle Family for use in minimally invasive spine surgery. The acquisition is highly complementary to Stryker’s current spine product portfolio and is aligned with the Spine division’s strategy of expanding its product offering for […]
Globus Medical won’t be deposed in patent infringement lawsuit
PHILADELPHIA (http://pennrecord.com) – An Audubon-based medical device manufacturer will not be forced to provide documents or give deposition testimony in a patent infringement lawsuit originating in federal court in Texas. In January, Globus Medical, Inc., a company that produces musculoskeletal implants to aid patients recovering from spinal injuries, received a subpoena from a Hawaii-based physician […]
Warsaw-based company accelerates medical device industry
Last tuesday, a Warsaw-based company unveiled an initiative designed to accelerate the medical device industry.Dubbed the ‘orthopedic capital of the world,’ the city of Warsaw has committed to support OrthoWorx over the next six years to help fund its new entity. The root of this effort, called AcceLinx, will serve as a platform for entrepreneurs […]
Spine Rep of Tomorrow: Adaptation is Key
April 3, 2016– “…but the species that survives is the one that is able best to ADAPT and adjust to the changing environment in which it finds itself” said Darwin. Spine Market: Changing Environment In the recent years, the Spinal Industry has started a transformation .Governments and health insurers worldwide are implementing measures to control costs, public hospitals are operating […]
Top Emerging Trends Impacting the Spine Market
The top four emerging trends influencing the global spine surgery market according to Technavio’s healthcare and life sciences research analysts are: Increased use of surface-modified titanium in spinal implants The use of titanium to manufacture spinal implants has increased in recent years. Spinal implants such as cages, rods, screws, hooks, wires, plates, and bolts are […]
Preparing For The New EU Medical Device Regulations
By Ronald Boumans and Stewart Eisenhart, Emergo–When European lawmakers and regulators first indicated plans to overhaul legislation on how the union oversees medical devices and in vitro diagnostics (IVDs) in 2010 and 2011, industry participants knew such changes to the world’s second-largest device market would be anything but sudden. Indeed, the wait for final legislation, […]
NuVasive – Same Opportunities, Same Concerns
(Seekingalpha.com)–Not a lot has really changed for NuVasive (NASDAQ:NUVA). The third-largest spine company in the U.S. by market share, NuVasive continues to take share from larger players on the back of innovative products that offer meaningful advantages in terms of patient outcomes and surgeon convenience. On the other hand, concerns remain about the company’s weaker […]
NJ Doctor Escapes Most Claims In Suit Over Spinal Implants
Law360, New York (March 2016, ) — A New Jersey doctor escaped most of the claims brought against him in federal court by a patient who accused him of medical malpractice after devices implanted in her spine failed, but he’s still facing negligence charges in the heavily trimmed case. U.S. District Judge Noel L. Hillman […]
Add our Apple Icon to your Desktop
If you visit our website frequently we recommend you to bookmark our site so that you can access to it without typing in the web address every time. However, you can quickly and easily access www.TheSPINEMarketGroup.com by adding an icon directly on the iPhone or iPad Home screen. How to do it? To add an Apple Icon to the desktop of an iPad or […]
Thank you INPAKOMED for the PLATINUM Sponsorship 2016
Thank you INPAKOMED! On behalf of TheSPINEMarketGroup team, we would like to thank you for your PLATINUM Sponsorship and for your support this year 2016. Inpakomed is a Contract Manufacturing Organization offering Medical Device Assembling and Packaging services within our ISO 13485 certified facilities. We offer a full range of services in our state-of-the art […]
Thank you MAROX for the PLATINUM Sponsorship 2016
Thank you MAROX! On behalf of TheSPINEMarketGroup team, we would like to thank you for your PLATINUM Sponsorship and for your support this year 2016. Marox is a leader in precision-machined medical device manufacturing, dedicated to smarter, more efficient and effective approaches to advanced manufacturing. Offering complete solutions and scalable services, we integrate manufacturing insight […]
DePuy Synthes Companies Announces New Strategic Alliance with Experts in Bundled Payments
ORLANDO, FL –March 2, 2016 – DePuy Synthes Companies* announced today a new exclusive strategic alliance with Value Stream Partners, LLC, experts in design, development and implementation of bundled payment programs for hip and knee replacements. The announcement was made here at the American Academy of Orthopaedic Surgeons (AAOS) 2016 Annual Meeting. “This exclusive arrangement […]
Atlas Spine Licenses True Position® Pivoting Spacer System Patents to Spine Wave
JUPITER, FL–(Marketwired – February 29, 2016) – Medical device company Atlas Spine, Inc. today announced the exclusive license of its True Position® Pivoting Spacer system patents and transfer of related assets to Spine Wave, Inc. The novel and patented Center Pivot Point Technology of the True Position® Spacer provides a powerful and controlled mechanical advantage, […]
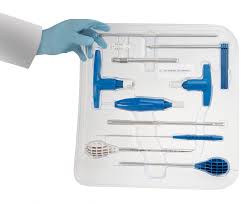
Single-use Sets are gaining traction in Spine
Single-use sets are gaining traction in Spine since their introduction in late 2014 . Physicians, like most human beings, are creatures of habit, preferring the familiar over the unknown. Most clinicians, for example, resisted the idea of electronic medical records in the late 1990s, deeming the time-saving tool an impersonal addition to their practices and an affront […]
Tyber Medical Announces a Statistically Significant Reduction of Bacteria
MORRISTOWN, N.J., Feb. 24, 2016 /PRNewswire/ — Tyber Medical, LLC, a privately held medical device company focused on developing innovative orthopedic and spine devices for private label opportunities, announces findings from research ongoing at Northeastern University that demonstrate a statistically significant reduction in bacteria on implant surfaces processed with BioTy™, a Modified Surface Treatment. The […]
Amedica to Present Advantages of Silicon Nitride Implants at 2016 Brazilian Spine Congress
SALT LAKE CITY, Feb. 23, 2016 (GLOBE NEWSWIRE) — Amedica Corporation (Nasdaq:AMDA), a company that develops and commercializes silicon nitride ceramics as a biomaterial platform, is pleased to announce its participation in the 2016 Brazilian Spine Congress occurring March 3-4, 2016 in Sao Paulo, Brazil. “Brazil has been an emerging growth opportunity for us since achieving […]