Dynam’X Taurus and related instruments set is designed for Transforaminal Lumbar Interbody Fusion (TLIF) procedure.
Benefits:
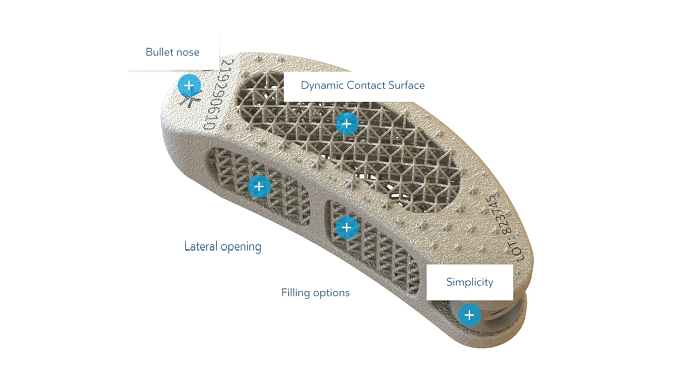
Dynam’X Taurus offers easy and straightforward TLIF implantation technique. Bulk-less implant holder with integrated movable implant joint module allows easy repositioning of implant. Due to light design Taurus cage can be applied to very collapsed disc space as for 7mm. Incorporated Syntropiq DCS ® Technology initiates and accelerates bone remodeling process, that results in short fusion time and massive creation of new bone scaffolds 1)
Dynam’X Taurus allows filling with bone substitute either by paste or granules formulations.
About Syntropiq
We are a privately held spine interbody implant and surface technology company. Our team members with rich experience more than 25 years in spine have seen over 10 000 spine surgeries and had several hundreds of hours of discussions with you – spine surgeons – to obtain much appreciated feedback on how to treat patients with spine disorders most successfully. Several hundreds of hours of discussions with spine surgeons created a solid connection between spinal biomechanics and cutting edge technologies implemented into innovative products.
Syntropiq manufactures and markets novel technology & devices for spinal interbody fusion procedure. Innovative application of biochanics and advanced additive manufacturing. We concentrate effort, energy and matter. We create complex spinal implants from the elemental titanium powder. We are Syntropiq.
Our Technologies:
- Iso Frame : Thanks to advanced engineering with Finite Elements Modeling the implementation IsoFrame Technology brings highest possible compatibility with diagnostics – specially in MRi environment. Optimized implant’s construct resulted significant reduction of metal volume, while keeping high durability. Due to IsoFrame also optimal balance between metal volume and endplate contact area has been achieved.
- Syntropiq Dynamic Contact Surface (DCS): On implants-bone contact area there is special controlled subsiding zone. Unique three dimensional design of Syntropiq DCS Mechanically initiates bone remodeling process1 and contributes proactively in acceleration of bone ingrown process.New bone structures growth process Is accelerated by engaging Wolf’s law. DCS employs controlled subsiding process and by following the cellular mechanism of bone remodeling initiates bone cells reversal process.. Engaging Wol’s Law accelerates process of new bone scaffolds growth.
- Additive Manufacturing from Titanium Powder: Titanium creates direct interaction between an implant and bone – the process is called osseointegration. Additive Manufacturing gives great possibility to control and precise engineer implant surface. The porous properties of titanium surface contributes to extensive bone infiltration, allowing osteoblast activity to take place (osteointegration). The porous structure allows also soft tissue adherence and vascularization within the implant.
Please for more information visit: http://www.syntropiq.com
