Kalamazoo, Michigan , Jan. 31, 2023 (GLOBE NEWSWIRE) — Stryker (NYSE:SYK) reported operating results for the fourth quarter and full year of 2022:
Fourth Quarter Results
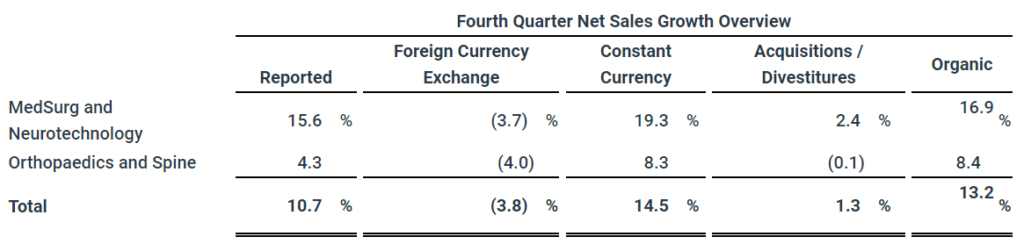
- Reported net sales increased 10.7% to $5.2 billion
- Organic net sales increased 13.2%
- Reported operating income margin of 15.6%
- Adjusted operating income margin(1) contracted 70 bps to 26.6%
- Reported EPS decreased 15.0% to $1.47
- Adjusted EPS(1) increased 10.7% to $3.00
Full Year Results
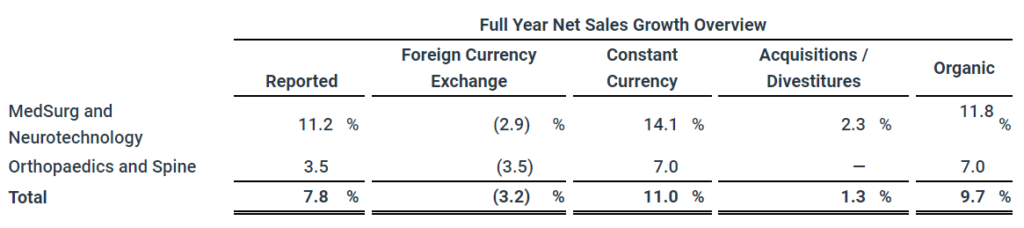
- Reported net sales increased 7.8% to $18.4 billion
- Organic net sales increased 9.7%
- Reported operating income margin of 15.4%
- Adjusted operating income margin(1) contracted 180 bps to 23.8%
- Reported EPS increased 18.4% to $6.17
- Adjusted EPS(1) increased 2.8% to $9.34
“We delivered outstanding organic sales growth in the fourth quarter, driven by strong commercial execution and improved supply,” said Kevin Lobo, Chair & CEO. “We expect continued positive sales momentum in 2023 and for adjusted earnings to gradually improve over the course of the year.”
Sales Analysis
Consolidated net sales of $5.2 billion and $18.4 billion increased 10.7% in the quarter, 14.5% in constant currency, and increased 7.8% in the full year, 11.0% in constant currency. Organic net sales increased 13.2% and 9.7% in the quarter and full year including 13.8% and 10.6% from increased unit volume partially offset by 0.6% and 0.9% from lower prices.
MedSurg and Neurotechnology net sales of $3.1 billion and $10.6 billion increased 15.6% in the quarter, 19.3% in constant currency, and increased 11.2% in the full year, 14.1% in constant currency. Organic net sales increased 16.9% and 11.8% in the quarter and full year including 15.7% and 11.2% from increased unit volume and 1.2% and 0.6% from higher prices.
Orthopaedics and Spine net sales of $2.2 billion and $7.8 billion increased 4.3% in the quarter, 8.3% in constant currency, and increased 3.5% in the full year, 7.0% in constant currency. Organic net sales increased 8.4% and 7.0% in the quarter and full year including 11.4% and 9.9% from increased unit volume partially offset by 3.0% and 2.9% from lower prices.
Earnings Analysis
Reported net earnings of $563 million and $2.4 billion decreased 15.0% and increased 18.3% in the quarter and full year. Reported net earnings per diluted share of $1.47 and $6.17 decreased 15.0% in the quarter and increased 18.4% in the full year. Reported gross profit margin and reported operating income margin were 62.2% and 15.6% in the quarter and 62.8% and 15.4% in the full year. In the quarter, we recorded a goodwill impairment charge of $216 million related to our Spine business. Reported net earnings include certain items, such as charges for acquisition and integration-related activities, the amortization of purchased intangible assets, asset write-offs and impairments and restructuring-related and other charges, costs to comply with certain medical device regulations, recall-related matters, regulatory and legal matters and tax matters. Excluding the aforementioned items, adjusted gross profit margin(1) was 62.7% and 63.1% in the quarter and full year, and adjusted operating income margin(1) was 26.6% and 23.8% in the quarter and full year. Adjusted net earnings(1) of $1.1 billion and $3.6 billion increased 11.1% and 2.8% in the quarter and full year. Adjusted net earnings per diluted share(1) of $3.00 and $9.34 increased 10.7% and 2.8% in the quarter and full year.
2023 Outlook
As we assess the current operating environment, we believe that there will continue to be macro-economic volatility caused by alleviating supply chain disruptions, inflationary risks and currency fluctuations. Despite the volatile macro-economic environment, we have good momentum in many parts of our business heading into 2023. We expect 2023 organic net sales growth(2) to be in the range of 7.0% to 8.5% and expect adjusted net earnings per diluted share(2) to be in the range of $9.85 to $10.15. Based on the steady progress of our pricing actions, we expect the impact of price to be between 0% and -0.5%. If foreign exchange rates hold near their current levels, we anticipate sales and EPS will be modestly unfavorably impacted as compared to 2022.
(1) A reconciliation of the non-GAAP financial measures: adjusted gross profit margin, adjusted operating income and adjusted operating income margin, adjusted net earnings and adjusted net earnings per diluted share, to the most directly comparable GAAP measures: gross profit margin, operating income and operating income margin, net earnings and net earnings per diluted share, and other important information accompanies this press release.
(2) We are unable to present a quantitative reconciliation of our expected net sales growth to expected organic net sales growth as we are unable to predict with reasonable certainty and without unreasonable effort the impact and timing of acquisitions and divestitures and the impact of foreign currency exchange rates. We are unable to present a quantitative reconciliation of our expected net earnings per diluted share to expected adjusted net earnings per diluted share as we are unable to predict with reasonable certainty and without unreasonable effort the impact and timing of restructuring-related and other charges, acquisition-related expenses and fair value adjustments to inventory and the outcome of certain regulatory, legal and tax matters. The financial impact of these items is uncertain and is dependent on various factors, including timing, and could be material to our Consolidated Statements of Earnings.
Conference Call on Tuesday, January 31, 2023
As previously announced, we will host a conference call on Tuesday, January 31, 2023 at 4:30 p.m., Eastern Time, to discuss our operating results for the quarter and year ended December 31, 2022 and provide an operational update.
Please register for this conference call at: Stryker’s Q4 and Full Year 2022 Earnings Call. After registering, a confirmation will be sent via email, including dial-in details and unique conference call access codes required for call entry. Registration is open throughout the live call. To ensure you are connected prior to the beginning of the call, we suggest registering a minimum of 15 minutes before the start of the call.
A simultaneous webcast of the call will be accessible via the Investor Relations page of our website at www.stryker.com. For those not planning to ask a question of management, we recommend listening via the webcast. Please allow 15 minutes to register, download and install any necessary software.
Following the conference call, a replay will be available at (866) 813-9403 (Toll Free) or (929) 458-6194 (International). The replay passcode is 332859. An archive of the webcast will also be available on our website two hours after the live call ends.
Caution Concerning Forward-Looking Statements
This press release contains information that includes or is based on forward-looking statements within the meaning of the federal securities law that are subject to various risks and uncertainties that could cause our actual results to differ materially from those expressed or implied in such statements. Such factors include, but are not limited to: the impact on our operations and financial results of the COVID-19 pandemic and any related policies and actions by governments or other third parties; weakening of economic conditions that could adversely affect the level of demand for our products; pricing pressures generally, including cost-containment measures that could adversely affect the price of or demand for our products; changes in foreign currency exchange markets; legislative and regulatory actions; unanticipated issues arising in connection with clinical studies and otherwise that affect United States Food and Drug Administration approval of new products; inflationary pressures; supply chain disruptions; changes in reimbursement levels from third-party payors; a significant increase in product liability claims; the ultimate total cost with respect to recall-related matters; the impact of investigative and legal proceedings and compliance risks; resolution of tax audits; the impact of the federal legislation to reform the United States healthcare system; costs to comply with medical device regulations; changes in financial markets; changes in the competitive environment; our ability to integrate and realize the anticipated benefits of acquisitions in full or at all or within the expected timeframes, including the acquisition of Vocera; our ability to realize anticipated cost savings; and potential negative impacts resulting from environmental, social and governance (ESG) and sustainability related matters. Additional information concerning these and other factors is contained in our filings with the United States Securities and Exchange Commission, including our Annual Report on Form 10-K and Quarterly Reports on Form 10-Q. We disclaim any intention or obligation to publicly update or revise any forward-looking statement to reflect any change in our expectations or in events, conditions or circumstances on which those expectations may be based, or that affect the likelihood that actual results will differ from those contained in the forward-looking statements.
Stryker is one of the world’s leading medical technology companies and, together with its customers, is driven to make healthcare better. The company offers innovative products and services in Medical and Surgical, Neurotechnology, Orthopaedics and Spine that help improve patient and healthcare outcomes. Alongside its customers around the world, Stryker impacts more than 130 million patients annually. More information is available at www.stryker.com.
For investor inquiries please contact:
Jason Beach, Vice President, Investor Relations at 269-385-2600 or [email protected]
For media inquiries please contact:
Yin Becker, Vice President, Chief Corporate Affairs Officer at 269-385-2600 or [email protected]
