SpineGuard (Paris:ALSGD) (FR0011464452 – ALSGD), an innovative company that deploys its DSG® (Dynamic Surgical Guidance) sensing technology to secure and streamline the placement of bone implants, reported today its full-year 2019 financial results as approved by the Board of Directors on March 19, 2020.
Pierre Jérôme, co-founder, Chairman and CEO of SpineGuard, said: “The reorganization plan we initiated mid-2017 and the efforts made since then allowed us to reach breakeven while continuing to deploy our real time sensing technology in new applications aimed at securing bone implant placement. Thanks to our financial discipline and compelling technological advances, we managed to obtain safeguard protection in France and Chapter 11 protection in the US. This favorable legal framework will enable the amendment of our debt. In parallel, we are actively pursuing our ambitious innovation strategy and the set-up of industrial partnerships on a sound basis in a context which is obviously significantly impacted by the propagation of the COVID-19 virus, for the very short term at least.”
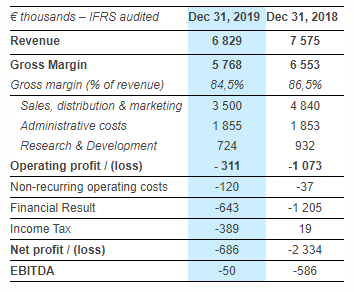
EBITDA improved by 91% and is positive in the second half-year
EBITDA improved by 91% at € -50k compared to € -586k in 2018.
EBITDA was € 301k positive in the second half of FY2019.
The current operating loss was reduced by 71% to € – 311k (vs. € -1,073k in 2018).
Gross margin decreased by 200 bps at 84.5% mainly in reason of adjusted manufacturing costs.
Operating expenses decreased by 20% or € 1,546k reflecting both the full impact of the company reorganization and a rigorous control of operational expenses over the year.
Operating cash flow was positive at € 224k (vs. € -669k in 2018).
At Dec. 31, 2019, cash and cash equivalents were € 1.3M, plus the secured € 0.7M of convertible bonds (OCAPI) for a total of € 2.0M. Working capital requirements were € 512k vs. € 782k in 2018.
Financial expenses mainly correspond to the interest charges of the venture loans with, Norgine Venture and Harbert European Growth Capital. There are € 148k of non-cash financial income related to the compliance with IFRS accounting principles on financing instruments.
2019: the strategic turn continues to deliver results
Robotics: in 2018, the company materialized two critical milestones for the smooth integration of DSG technology into surgical robots. In 2019, SpineGuard successfully completed a feasibility study in collaboration with the laboratory ‘Institut des Systèmes Intelligents et de Robotique’ of the Paris Sorbonne University (ISIR). This experiment demonstrated how DSG technology stops a surgical robot automatically when an impending bone breach is detected. It also highlighted the efficacy and adequacy of DSG as applied to robotic surgeries in order to automate breach detection. The long-term goal is to automate skeletal implant placements. These achievements also provided ground for IP and were also showcased to potential industrial strategic partners in collaboration with Healthios Capital Markets, the US bank assisting the company in that purpose.
Visualization of the signal (DSG Connect): in 2019, SpineGuard finalized its next generation of products ‘PediGuard DSG Connect’. They will be enabled by a visualization software embedded into a tablet coupled with ‘bluetooth like’ technology. The Company filed its CE mark dossier at the end of September 2019 and progressed in parallel with its 510k filing to be submitted to the FDA in the first half of 2020. The company is aiming at pre-commercial launch in Europe and the USA in the course of 2020.
Dental Implantology (worldwide licensing): the pertinence of DSG technology beyond spine has been demonstrated by the CE mark and the manufacturing of the first generation of products (‘SafeGuard’) dedicated to the dental market. The partnership with ConfiDent ABC (Adin Group) in the context of the worldwide licensing agreement continues with great momentum.
2020 PERSPECTIVES
The collaboration with our industry partner Adin Dental/ ConfiDent on the dental application will intensify in 2020 with the co-development of a next generation DSG embedded product, fruit of the feedback on the first generation tested in 2019.
The search for strategic alliances with industry players notably for the robotic application continues with the venture bank Healthios Capital Markets.
Lastly, the DSG Connect platform should soon be CE Marked cleared and the FDA filing is progressing. This strategic high-value innovation is already used experimentally by the company to guide surgical robots. It will be deployed to the full PediGuard range and Smart Screw products. The company believes that it will bring a renewed sales momentum in particular in Europe and the USA, but also in other high potential geographies such as Brazil, Saudi Arabia and Turkey. Of course, the pace will highly depend on the containment of the COVID-19 outbreak and the resuming of a normal activity in the hospitals. For now, in a growing number of countries, most of the elective surgeries are being postponed to prepare for and focus on COVID-19 patients.
Additional information on “safeguard” and Chapter 11 proceedings
SpineGuard is planning to release early next week updated information regarding the ongoing progress of its voluntary filings made mid-February, in France and the USA, under the ‘safeguard’ and Chapter 11 procedures. On this occasion, SpineGuard may communicate regarding the resuming of trading on Euronext.
Next financial press release: 2020 first quarter revenue on April 23, 2020
About SpineGuard®
Founded in 2009 in France and the USA by Pierre Jérôme and Stéphane Bette, SpineGuard is an innovative company deploying its proprietary radiation-free real time sensing technology DSG® (Dynamic Surgical Guidance) to secure and streamline the placement of implants in the skeleton. SpineGuard designs, develops and markets medical devices that have been used in over 75,000 surgical procedures worldwide. Fifteen studies published in peer-reviewed scientific journals have demonstrated the multiple benefits DSG® offers to patients, surgeons, surgical staff and hospitals. Building on these solid fundamentals and several strategic partnerships, SpineGuard has expanded its technology platform in a disruptive innovation: the « smart » pedicle screw launched late 2017 and is broadening the scope of applications in dental implantology and surgical robotics. DSG® was co-invented by Maurice Bourlion, Ph.D., Ciaran Bolger, M.D., Ph.D., and Alain Vanquaethem, Biomedical Engineer.
For further information, visit www.spineguard.com
Disclaimer
The SpineGuard securities may not be offered or sold in the United States as they have not been and will not be registered under the Securities Act or any United States state securities laws, and SpineGuard does not intend to make a public offer of its securities in the United States. This is an announcement and not a prospectus, and the information contained herein does and shall not constitute an offer to sell or the solicitation of an offer to buy, nor shall there be any sale of the securities referred to herein in the United States in which such offer, solicitation or sale would be unlawful prior to registration or exemption from registration.
Contacts
SpineGuard
Pierre Jérôme
Chairman and CEO
Tél. : +33 1 45 18 45 19
[email protected]
Manuel Lanfossi
Chief Financial Officer
[email protected]
Europe / NewCap
Investor Relations & Financial Communication
Mathilde Bohin / Pierre Laurent
Tél. : +33 1 44 71 94 94
[email protected]
