SpineGuard (FR0011464452 – ALSGD) (Paris:ALSGD), an innovative company that deploys its DSG® (Dynamic Surgical Guidance) sensing technology to secure and streamline the placement of bone implants, announced today financial results for the half year ending June 30, 2022, as approved by the Board of Directors on September 15, 2022.
Pierre JEROME, cofounder, Chairman & CEO of SpineGuard, said: “These half year results are in line with our financial objectives. They reflect our strategy to invest selectively in both the strengthening of our US commercial structure and the further deployment of our DSG technology while remaining close to break-even and minimizing dilution for our shareholders. Our cash runway and growth prospects, in particular those related to the two new strategic partnerships initiated this year, enable us to be confident and enthusiastic about SpineGuard’s future.”
EBITDA improves by 15%
2,848 DSG units were sold in the first half of 2021 compared with 2,731 in the first half of 2021, including 1,340 in the United States, representing 47% of total units sold.
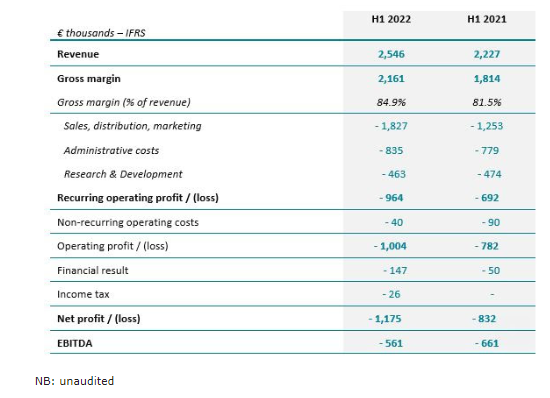
Revenue was still impacted by the COVID-19 pandemic early 2022. For H1 2022, the Company reported revenues of € 2,546 K, up 14% as reported in H1 2021 and +6.5% in constant currency compared with H1 2021.
Gross margin of 84.9% at June 30, 2022 improved by 3.9%. ASP’s remained globally stable and while the company faced some higher costs of manufacturing, they remained limited overall. The return to growth also reduced the impact of the scraps of obsolete inventory in the USA incurred in 2021.
Operating expenses were € 3,131 K for H1 2022, compared with € 2,506 K, an increase of € 625 K compared with June 30, 2021 driven by the momentum of sales & marketing initiatives in the US.
The net operating loss stands at € -964 K vs. € -692 K at June 30, 2021.
EBITDA improved 15% at € -561 K compared to € -661 K at June 30, 2021.
Working capital requirements were € 247 K compared with € -133 K at December 31, 2021 mainly due to the increase of account receivables (+€ 271 K) and inventory (+€ 141 K).
At June 30, 2022, cash and cash equivalents were € 4,450 K compared with € 5,207 K at December 31, 2021, and is explained as follows:
- Operating cash flow of € -921 K compared with the same period last year of € -620 K.
- Equity funding using the equity lines for a gross amount of € 1,006 K throughout the period.
- The payment of interests to Norgine Ventures and Harbert European Growth of € 157 K.
- Reimbursement of the principal of the venture loans for € 336 K over the half-year.
The cash position as of August 31, 2022 of € 3.7 M plus the secured equity line facility for € 4.0 M means that the total cash available to the Company is € 7.7 M. Considering the current cash position, the secured convertible bond facility and the recurring expected business, the Company estimates that it can fund its needs through mid-2024.
Post-closing events
SpineGuard and Omnia Medical, a medical device company focused on innovative solutions utilizing proven techniques, announced the signature of a co-development and exclusive distribution agreement for adult spine surgery in the United States. This partnership spans two novel devices: a smart single-step pedicle screw system and a smart drilling tool for sacroiliac joint fusion both embedding the DSG (Dynamic Surgical Guidance) technology.
SpineGuard’s Priorities
SpineGuard is focusing on the following priorities while investing selectively to remain close to breakeven:
- Boost commercial activities with the launch of the DSG-Connect visual interface and the partnership with WishBone Medical
- Deploy the DSG digital technology in the surgical robotic field
- Develop a Smart Universal Drill (SUD) embedding the DSG artificial intelligence
- Support ConfiDent with the design and scale-up of the DSG dental applications
- Implement the agreement recently signed with Omnia Medical
- Sign other strategic partnerships
The company’s half-year financial report is available in the Investors > Exchange filings section of the www.spineguard.com website in French only.
Next financial press release: Third quarter 2022 revenue on October 12, 2022, after market closing.
About SpineGuard®
Founded in 2009 in France and the USA by Pierre Jérôme and Stéphane Bette, SpineGuard is an innovative company deploying its proprietary radiation-free real time sensing technology DSG® (Dynamic Surgical Guidance) to secure and streamline the placement of implants in the skeleton. SpineGuard designs, develops and markets medical devices that have been used in over 90,000 surgical procedures worldwide. Nineteen studies published in peer-reviewed scientific journals have demonstrated the multiple benefits DSG® offers to patients, surgeons, surgical staff and hospitals. Building on these strong fundamentals and several strategic partnerships, SpineGuard has expanded the scope of its DSG® technology in innovative applications such as the « smart » pedicle screw, the DSG Connect visualization and registration interface, dental implantology and surgical robotics. DSG® was co-invented by Maurice Bourlion, Ph.D., Ciaran Bolger, M.D., Ph.D., and Alain Vanquaethem, Biomedical Engineer. SpineGuard has engaged in multiple ESG initiatives.
For further information, visit www.spineguard.com
Disclaimer
The SpineGuard securities may not be offered or sold in the United States as they have not been and will not be registered under the Securities Act or any United States state securities laws, and SpineGuard does not intend to make a public offer of its securities in the United States. This is an announcement and not a prospectus, and the information contained herein does and shall not constitute an offer to sell or the solicitation of an offer to buy, nor shall there be any sale of the securities referred to herein in the United States in which such offer, solicitation or sale would be unlawful prior to registration or exemption from registration.
Contacts
NewCap
Mathilde Bohin / Aurélie Manavarere
Investor Relations & Strategic Communication
Phone nb.: 01 44 71 94 94
Email: [email protected]
SpineGuard
Pierre Jérôme
Chairman and CEO
Phone nb.: 01 45 18 45 19
Email: [email protected]
