PARIS and BOULDER (CO), July 12, 2022 – 6:00 pm CEST – SpineGuard (FR0011464452 – ALSGD), an innovative company that deploys its DSG® (Dynamic Surgical Guidance) sensing technology to secure and streamline the placement of bone implants, announced today its first half 2022 revenue.
Pierre Jérôme, Co-founder, Chairman and CEO of SpineGuard, declared: “Driven by the rebound of our commercial activities in the US and the increasing impact of our new DSG Connect platform on the heels of a sustained performance in Europe and Latin America, our sales growth is accelerating in Q2. The feedback from the 20+ centers having evaluated DSG Connect is highlighting its value added in particular with regards to shortening the learning curve for the new adopters of our x-ray free real time Dynamic Surgical Guidance technology. We have scheduled its full-scale launch in October around the NASS (North American Spine Society) and Eurospine congresses, the two main annual events in our industry. On another note, we are very satisfied with the first six months of our collaboration with WishBone Medical, our new strategic partner. The contractual agreements they have put in place with US hospital GPOs (Group Purchasing Organizations) definitively provide them with quick access to the pediatric orthopedic market.“
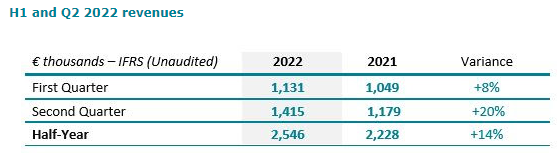
In the United States, the revenue of the second quarter 2022 grew by 19% to $ 1,105K from $ 1,054K (+5% cc).
Outside the USA, the revenue of the second quarter grew by +24% at € 378K. The half-year revenue grew by +18% at € 770K.
2,848 DSG units were sold in the first half of 2022 from 2,731 units in first half of 2021, a growth of 4% (+9% on the second quarter alone). 1,340 units were sold in the United States representing 47% of total units.
Cash position
At June 30, 2022, cash and cash equivalents were € 4.5M. Including the € 4.0M balance of the Nice & Green convertible warrants (BSAR) equity line, SpineGuard’s cash runway stands until Q2-2024.
In addition, should 100% of the Warrants be exercised, the free allocation of Redeemable Warrants set up in June 2021 may generate gross proceeds of € 5,338K and would extend the cash runway by 24 additional months.
2022 Perspectives
SpineGuard is focusing on the following priorities while investing selectively to remain close to breakeven:
- Boost commercial activities with the launch of the DSG-Connect visual interface and the partnership with WishBone Medical
- Deploy the DSG digital technology in the surgical robotic field
- Develop a Smart Universal Drill (SUD) embedding the DSG artificial intelligence
- Support ConfiDent with the design and scale-up of the DSG dental applications
- Sign new strategic partnerships
Next financial press release: 2022 Half-year financial results on September 14, 2022
About SpineGuard®
Founded in 2009 in France and the USA by Pierre Jérôme and Stéphane Bette, SpineGuard is an innovative company deploying its proprietary radiation-free real time sensing technology DSG® (Dynamic Surgical Guidance) to secure and streamline the placement of implants in the skeleton. SpineGuard designs, develops and markets medical devices that have been used in over 90,000 surgical procedures worldwide. Nineteen studies published in peer-reviewed scientific journals have demonstrated the multiple benefits DSG® offers to patients, surgeons, surgical staff and hospitals. Building on these strong fundamentals and several strategic partnerships, SpineGuard has expanded the scope of its DSG® technology in innovative applications such as the « smart » pedicle screw, the DSG Connect visualization and registration interface, dental implantology and surgical robotics. DSG® was co-invented by Maurice Bourlion, Ph.D., Ciaran Bolger, M.D., Ph.D., and Alain Vanquaethem, Biomedical Engineer. SpineGuard has engaged in multiple ESG initiatives.
For further information, visit www.spineguard.com
Disclaimer
The SpineGuard securities may not be offered or sold in the United States as they have not been and will not be registered under the Securities Act or any United States state securities laws, and SpineGuard does not intend to make a public offer of its securities in the United States. This is an announcement and not a prospectus, and the information contained herein does and shall not constitute an offer to sell or the solicitation of an offer to buy, nor shall there be any sale of the securities referred to herein in the United States in which such offer, solicitation or sale would be unlawful prior to registration or exemption from registration.
Contacts
SpineGuard
Pierre Jérôme
CEO & Chairman
Tel: +33 1 45 18 45 19
[email protected]
SpineGuard
Manuel Lanfossi
CFO
Tel: +33 1 45 18 45 19
[email protected]
NewCap
Investor Relations & Financial Communication
Mathilde Bohin
Tel: +33 1 44 71 94 94
[email protected]
