PARIS and BOULDER (CO), October 11, 2023 – 6:00 pm CEST – SpineGuard (FR0011464452 – ALSGD), an innovative company that deploys its DSG® (Dynamic Surgical Guidance) sensing technology to secure and streamline the placement of bone implants, announced today its third quarter 2023 revenue.
Pierre Jérôme, Co-founder, Chairman and CEO of SpineGuard, said: “Despite our revenue decline in the last 6 months, this quarter marks the rebound of SpineGuard sales in the United States with a sequential growth of 19% over the second quarter. The newly set-up American commercial team is ramping-up with focus on bolstering our agent network and promoting the recently launched products, PediGuard Threaded and DSG Connect, which have been significantly contributing to the steady increase of our product sales over the last ten quarters or so in the rest of the world, +26% year-to-date. In parallel, we are finalizing the development of three new products that we plan to introduce on the market within the next twelve months in order to swiftly get back to double digit global growth and continue to expand the application scope of our DSG technology.”
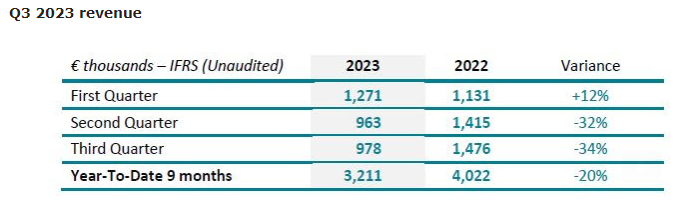
SpineGuard’s consolidated revenue decreased by -34% in Q3 2023 compared to Q3 2022. In the United States, the revenue of the third quarter 2023 decreased by 29% in USD, to $ 728K from $ 1,020K. This is mainly due to the low level of orders from SpineGuard’s main American distributor, which is experiencing its own financial and governance difficulties. However, a recovery in business has been observed under the impetus of the renewed American team. The third-quarter sales are up by 19% to $ 728K vs. $ 610K in the second quarter. In the rest of the world, the product sales of the third quarter 2023 grew by +5% for the products and decreased by 33% when factoring in the interruption of royalties revenue related to the dental project. 4,395 DSG units were sold in the first nine months of 2023 vs. 4,209 units in first nine months of 2022, which is a global growth of +4%. 1,596 units were sold in the United States representing 36% of total units.
Cash position
At September 30, 2023, cash and cash equivalents were € 4.0M. To continue bolstering its equity, the company is currently considering other funding opportunities, including a capital increase with retention of preferential subscription rights, rather than to use the Horizon equity line put in place on May 31, 2023 with Nice & Green’s for an amount of € 7.5M, which remains undrawn at the date of this press release.
Perspectives
SpineGuard is focusing on the following priorities while investing selectively and with rigor:
- Boost commercial activities with PediGuard Threaded and the DSG-Connect platform
- Develop the DSG drill bit for a launch in 2024
- Codevelop the Sacro-Iliac DSG device and Smart Screw with Omnia Medical
- Implement the three-prong agreement recently signed with XinRong Medical for China
- Deploy the DSG technology in the surgical robotic and dental fields
- Initiate new strategic partnerships
About SpineGuard®
Founded in 2009 in France and the USA by Pierre Jérôme and Stéphane Bette, SpineGuard is an innovative company deploying its proprietary radiation-free real time sensing technology DSG® (Dynamic Surgical Guidance) to secure and streamline the placement of implants in the skeleton. SpineGuard designs, develops and markets medical devices that have been used in over 95,000 surgical procedures worldwide. Twenty-five studies published in peer-reviewed scientific journals have demonstrated the multiple benefits DSG® offers to patients, surgeons, surgical staff and hospitals. Building on these strong fundamentals and several strategic partnerships, SpineGuard has expanded the scope of its DSG® technology in innovative applications such as the « smart » pedicle screw, the DSG Connect visualization and registration interface, dental implantology and surgical robotics. DSG® was co-invented by Maurice Bourlion, Ph.D., Ciaran Bolger, M.D., Ph.D., and Alain Vanquaethem, Biomedical Engineer. SpineGuard has engaged in multiple ESG initiatives.
For further information, visit www.spineguard.com
