In today’s spine market, there are more than 400 competing companies. Some of them are well known for being market leaders and others have gradually gained greater relevance. However, there are still many companies that have interesting products that are quite unknown to people. Today we want to present four companies that, either for having a unique product or for having an attractive portfolio, we consider it worthwhile to be better known:
1.-AMB SURGICAL II
AMB Surgical is an early-stage medical device product developer that holds two issued patents for an electro-mechanical smart automated growing rod called “FLYTE”. FLYTE™ has the potential to revolutionize the way pediatric deformities are treated and spare young patients repeated, invasive procedures. FLYTE’s technology can advance surgical techniques and outcomes for complex applications in pediatric scoliosis, complex deformity correction (dynamic external fixation/ X-Fix), and long bone lengthening (intramedullary nail applications]. FLYTE delivers precise, frequent, less traumatic adjustments, along with providing data capture/storage and biomechanical feedback. The result is a reduction in costs and surgical risks and improved patient outcomes.
To Highlight: The FLYTE smart automated growing rod technology. FLYTE is a sophisticated electro-mechanical, software activated device to provide real-time biomechanical feedback to guide treatment, as well as, allow surgeons to perform accurate, non-surgical skeletal adjustments. Through our work, AMB Surgical is advancing the boundaries of modern medicinal practices.http://ambsurgical.com
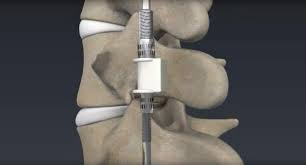
2.-NEXUS SPINE
Nexus Spinal has produced a line of patented, ultra low profile pedicle screws that are ideally suited for new, minimally invasive approaches to lumbar spinal fusion. These unique, new products are aimed at reducing the trauma to soft tissues that occurs from surgery. http://www.nexusspine.com
To Highlight: Their lumbar system PressON Pro offers a dependable solution using only 3 instruments in a fraction of the time. There is no rod bending or set screw needed.

3.-CURITEVA
Headquartered in Huntsville, Alabama, and founded in 2018, Curiteva is a global medical technology company dedicated to improving the quality of life for patients by providing innovative and intuitive spinal implant systems. Curiteva is a rapidly growing company engaged in research and development, product design, manufacturing, quality control and marketing of spinal implants and related products.Website: https://www.curiteva.com
To Highlight: The Prodigy MIS Pedicle Screw System:The Prodigy MIS Pedicle Screw System is designed to minimize the complexity of procedural steps while correcting and stabilizing the posterior thoracolumbar spine through a percutaneous approach. This system provides the same strength and rigidity as the Prodigy Polyaxial Screw System.
4.-NeuroPro Spinal Jaxx, Inc.
It is a research, development and distribution company specializing in next generation expandable interbody fusion devices. Spinal Jaxx also comes with a best-in-class biomaterial on bone-contacting surfaces. Current products support TLIF and PLIF approaches. ALIF, XLIF and other implants are under development. Devices with lordotic angles are also under development. The Spinal Jaxx patented technology is a leap forward from the technology of competing expandables available on the market today. Current products have been cleared by the FDA. Website: www.spinaljaxx.com
To Highlight: Spinal Jaxx is an expandable lumbar fusion device intended for use in spinal fusion surgeries and allows surgeons to customize the implant to the specific anatomy of the patient. The proprietary instrumentation also allows the surgeon to effectively fill the implant with auto graft after implantation and set the implant to the desired expansion level.The device is made from titanium (endplates that contact the vertebral bodies) and PEEK (body of the implant). The surgeon also has the option of using a Spinal Jaxx version that includes a unique titanium scaffold with interconnected pores on the endplates for a superior interface with the bone.

