CARLSBAD, CA (PRWEB) AUGUST 01, 2016–Spinal Elements, a spine technology company, announced that over 10,000 Ti-Bond interbody devices have been implanted and to celebrate this milestone a Ti-Bond Warranty Program has been initiated. Ti-Bond is the name of Spinal Elements’ porous titanium coating that is applied to PEEK interbody devices used in spine surgery. The company was a pioneer in this type of implant and the coating is available on the wide variety of PEEK interbody devices the company offers.
PEEK (poly-ether-ether-ketone) has been used in spine interbody devices for over 15 years. The material is radiolucent which is ideal for helping assess spinal fusion and has mechanical performance properties that make it ideal for the loads seen in the intervertebral space of the spine. PEEK, however, is hydrophobic meaning that it repels fluids. The Ti-Bond coating that Spinal Elements applies on the PEEK device at the endplates is hydrophilic, meaning that it attracts fluids. Additionally, the coating is a roughend surface to be placed juxtaposed to the vertebrae of the spine.
Because of the overwhelmingly positive experience gained and confidence in the devices with Ti-Bond coating, Spinal Elements is now able to offer a warranty for its Ti-Bond implants. The devices are warrantied against delaminating, chipping, peeling, shedding, or generating debris at the time of surgery and are further warrantied against implant fracture in whole or in part, coating delamination, pseudarthrosis, implant migration, subsidence, or trauma for a year after surgery. The company will replace the implant free of charge if any of these events are to occur. Interested parties are encouraged to contact the company for more details about the Ti-Bond Warranty Program.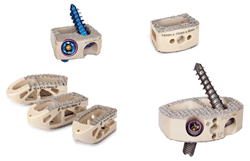
Todd Andres, CEO and co-founder of Spinal Elements, had this to say about the milestone and warranty: “Ti-Bond has been an important component of our growth since its launch, fueling our more than 20% growth year to date and near 30% growth in the most recent quarter for this year over last year alone. We see growing excitement for it as we continue to introduce Ti-Bond into new segments including lateral and expandable interbody devices. The Warranty Program serves as a statement of our phenomenal confidence in this technology to deliver the performance demanded by our surgeon customers.”
For more information about the warranty, view the company’s website at http://www.spinalelements.com or e-mail the company at warranty(at)spinalelements(dot)com.
About Spinal Elements
Spinal Elements, headquartered in Carlsbad, CA, is a spine technology company for spine surgeons who demand innovative, extremely high quality surgical solutions. From the company’s early work which helped make PEEK commonplace throughout the spine industry to recent advancements in Ti-Bond® porous titanium coated PEEK interbody implants and controlled delivery technology, Spinal Elements has built a reputation for being trustworthy, innovative and different. The company is focused on the development and marketing of progressive spinal treatment options and markets a complete portfolio of advanced spinal implant technologies. Additionally, the company distributes Hero® Allograft, the net proceeds from which are donated to charities benefitting children with life-threatening medical conditions. For more information, please visit http://www.spinalelements.com. Follow us on Twitter @SpinalElements and on LinkedIn for continuous company updates.
