Lyon (France), July 18, 2023 – 5.45 pm CEST – SMAIO (Software, Machines and Adaptative Implants in Orthopaedics – Euronext Growth Paris ISIN: FR0014005I80 / Ticker: ALSMA, eligible for PEA-PME equity savings plans), a French player specialized in complex spine surgery with a comprehensive offer including software, adaptative implants and related services, today published its sales for the first half of 2023 and issued an update on its development.
Philippe ROUSSOULY, Chairman and CEO of SMAIO, said: “The first half of 2023 was firstly marked by the achievement of a major milestone for SMAIO with the granting of FDA 510(k) clearance for a customized version of the KEOPS Balance Analyzer 3D surgery planning software co-developed with NuVasive. In accordance with the plan, this clearance triggered a $3 million payment from our American partner. This partnership is the cornerstone of our US market access strategy. Firstly, it demonstrates the capabilities of the technological collaboration between the two companies, and, secondly, it provides NuVasive clients, primarily in the United States, with the possibility of using our high-performance customized surgery planning services in the near future. These activities should thus create additional recurring revenue flows from the end of 2023 or early 2024, depending on registration timeframes in selected North American centers. These flows should help improve the Company’s profitability indicators and reduce its cash requirements.
Furthermore, during the semester, SMAIO has focused its commercial efforts on training ambassador surgeons on the American market for the dissemination of its comprehensive i-kontrol offer. Following registration procedures in targeted American hospitals, several complex surgeries could be performed during the second half of the year. At the same time, the confirmed participation of more than 30 surgeons specialized in complex surgery in the “Sagittal Alignment Think Tank” organized by SMAIO in November 2023 in San Diego is an extremely positive sign of the rapid ramping up of the Company’s reputation in the United States. Consequently, this targeted and controlled strategic investment policy, combined with the use of non-dilutive financing levers carried out in advantageous conditions with Bpifrance and the Company’s longstanding banking partners (BNP Paribas, Société Générale), enabled SMAIO to have a comfortable cash position at June 30, 2023. Within this context, the Company will continue its efforts to achieve the $2 million second milestone payment with NuVasive, further strengthening its partnership while significantly developing its sales and profitability”.
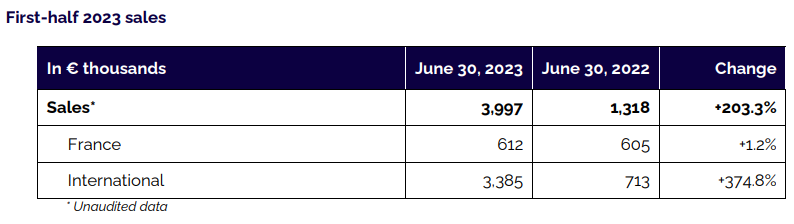
Sales totaled €4.0 million in the first half of 2023, up +203% compared with the first half of 2022 (€1.3m).
International activity saw very strong growth compared with the same period of 2022 (+375%). This substantial increase was primarily due to the $3 million (approx. €2.8 million) milestone payment received from NuVasive, a leading player in technological innovations for spine surgery, following the granting of FDA 510(k) clearance for a customized version of the surgery planning software co-developed with this partner, in line with the roadmap published at the time of the Company’s IPO. Moreover, sales of implants and instruments have recorded good growth via distributors in Spain and in Sweden, as well as a new distributor in Australia.
Excluding the NuVasive milestone payment, first-half 2023 revenue primarily consisted of sales of the Kheiron system comprising customized implants and rods (€1.2 million). Kheiron sales should be driven by the opening up of the US market in the second half of 2023, with a number of American centers currently in the final stages of the registration process for SMAIO’s implantable devices.
Regarding the software segment, the latter should increase over coming semesters thanks to the partnership and licensing agreement signed with NuVasive. Within this framework, SMAIO could provide its planning services to NuVasive’s clients and generate additional recurring revenue by the end of 2023 or early 2024, depending on the required registration timeframes in the targeted American hospitals.
Solid financial structure
The Company had a cash position of €7.3 million at June 30, 2023 compared with €5.7 million at December 31, 2022. This increase was due to good control over cash burn levels, the payment relating to the milestone achieved with NuVasive and the receipt of the €1 million Bpifrance Innovation R&D loan that includes a 3-year grace period.
Moreover, the Company’s cash position will benefit from the drawdown, in September, of €1.5 million in the form of loans from BNP Paribas and Société Générale. These loans have a maturity of 4 years.
This level of cash will allow SMAIO to finance its development in accordance with the strategy presented at the time of the Company’s IPO, in April 2022.
Upcoming financial publication H1 2023 results: Wednesday, October 18, 2023, after market
About SMAIO
A precursor in the use of clinical data and imaging of the spine, SMAIO designs global solutions for spine surgery specialists. The Company has recognized expertise thanks to KEOPS, its Big Data management software that has become a global reference with more than 100,000 patient cases documented.
SMAIO offers spine surgeons a comprehensive platform, I-Kontrol, incorporating planning, implants and related services, enabling them to treat spinal pathologies in a safe, effective and lasting way.
SMAIO is positioned at the forefront of innovation with the ambition of providing surgeons with the first active robotic solution enabling a high level of performance and repeatability to be achieved.
Based in Lyon, France, SMAIO benefits from the skill and expertise of more than 30 highly specialized staff.
For further information, please visit our website: www.smaio.com
SOURCE: https://www.smaio-finance.com/images/PDF/PR_SMAIO_18-07-2023_H1-2023_sales.pdf
