SMAIO (Software, Machines and Adaptative Implants in Orthopaedics – Euronext Growth Paris ISIN: FR0014005I80 / Ticker: ALSMA) (Paris:ALSMA), a French player specialized in complex spine surgery with a global offer comprising software, adaptative implants and related services, today published its sales for the first half of 2022.
Philippe ROUSSOULY, Chairman and CEO of SMAIO, commented: “We recorded a solid first half with sales growth of 28%. Our development was particularly dynamic abroad, where our sales more than doubled mainly thanks to the increased strength of our historical distributors in Spain and Scandinavia, new distribution agreements signed in the Baltic States and Greece, and to the first surgical procedures performed in the United States, illustrating the pertinence of the positioning of our unique and global solution in the field of spine surgery, i-Kontrol. Over the coming semesters, we will focus on continuing the development of our activity in France, Europe, Australia and the United States thanks to the organizing of training programs enabling the concepts, technologies and services provided by SMAIO to be highlighted. Our primary objective is to significantly accelerate our sales momentum on the US market, which strongly values technological innovations such as our patient-specific surgery solutions. The 510(k) clearance recently granted by the FDA for both our surgery planning software and our patient-specific rods represent the first steps in our ramping up on this key market. Furthermore, the collaboration with our American partner NuVasive should enable us to receive a first milestone payment of $3 million in early 2023 associated with the partnership contract signed between the two companies, which also provides for the implementation of recurrent revenue associated with imaging analysis services undertaken by SMAIO’s operators for NuVasive’s clients”.
First-half 2022 sales
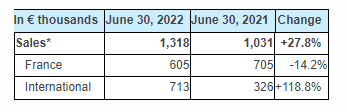
* Unaudited data
Sales totaled €1.3 million in the first half of 2022, an increase of +28% compared with the first half of 2021 (€1.0 million).
International activity accounted for over half of the Company’s sales (54%) and more than doubled (+119%) compared with the first six months of 2021. This buoyant increase was a result of the strengthening of our historical distributors in Spain and Scandinavia, new distribution agreements signed in the Baltic States and Greece, as well as the first surgeries performed in the United States.
In France, sales slipped to around €0.6 million. However, activity should remain strong over the coming months, driven by the intensification of the marketing of i-Kontrol solution to new medical facilities.
As was the case in 2021, sales of implants and rods accounted for almost all of the Company’s sales (96%). The software segment, with the Keops platform, accounted for the balance (4%) and will see substantial growth over the coming semesters, notably following the granting of 510(k) clearance by the FDA and thanks to the partnership and licensing agreement signed with NuVasive, a global leader in spine technology innovation.
Two recent 510(k) clearances to accelerate the distribution of the i-Kontrol solution in the USA
In June, SMAIO announced that it had been granted 510(k) clearance by the FDA for the key components of its i-Kontrol platform: the Balance Analyzer 3D surgery planning software and the K-rod patient-specific union rod. Thanks to these approvals, SMAIO can now offer its i-Kontrol solution to North American medical centers. These two approvals represent a major step in the American market penetration strategy that will be based on the distribution of the comprehensive i-Kontrol solution to key centers invited to participate in training programs called the “sagittal alignment academy”, as well as on imaging analysis services distributed via NuVasive’s network as soon as the first version of the SMAIO surgery planning software jointly developed with its California partner is approved.
Upcoming financial announcement:
- Publication of H1 2022 results: September 27, 2022, after market
About SMAIO
A precursor in the use of clinical data and imaging of the spine, SMAIO designs global solutions for spine surgery specialists. The Company has recognized expertise thanks to KEOPS, its Big Data management software that has become a global reference with more than 100,000 patient cases documented.
SMAIO offers spine surgeons a comprehensive platform, I-Kontrol, incorporating planning, implants and related services, enabling them to treat spinal pathologies in a safe, effective and lasting way.
SMAIO is positioned at the forefront of innovation with the ambition of providing surgeons with the first active robotic solution enabling a high level of performance and repeatability to be achieved.
Based in Lyon, France, SMAIO benefits from the skill and expertise of more than 30 highly specialized staff.
For further information, please visit our website: www.smaio.com
Listing market: Euronext Growth Paris
ISIN: FR0014005I80
Mnemonic: ALSMA
Disclaimer
This press release contains non-factual elements, including, but not limited to, certain statements regarding future results and other future events. These statements are based on the current vision and assumptions of the management of the Company. They incorporate known and unknown risks and uncertainties that could result in significant differences in results, profitability and expected events. In addition, SMAIO, its shareholders and its affiliates, directors, officers, counsels and employees have not verified the accuracy of, and make no representations or warranties about, statistical information or forecast information contained within this news release and that originates or is derived from third party sources or industry publications; these statistical data and forecast information are only used in this press release for information purposes. Finally, this press release may be drafted in French and in English. In the event of differences between the two texts, the French version will prevail.
Contacts
SMAIO
Philippe Roussouly
Chief Executive Officer
Renaut Fritsch
Chief Financial Officer
[email protected]
NewCap
Dusan Oresansky/Quentin Massé
Investor Relations
[email protected]
Tel.: +33 (0)1 44 71 94 92
NewCap
Nicolas Merigeau
Media Relations
[email protected]
Tel.: +33 (0)1 44 71 94 98
